��Ŀ����
��һƿ��ɫ��Һ�����ܺ���Na+��K+��Al3+��Mg2+��NH4+��Cl����SO42����HCO3����MnO4�������еļ��֡�Ϊȷ����ɷ֣���������ʵ�飺��ȡ������Һ�����������Na2O2���壬������ɫ��ζ������Ͱ�ɫ�����Ұ�ɫ������������ֲ����ܽ⣻����ȡ������Һ������HNO3�ữ��Ba(NO3)2��Һ���а�ɫ�������������ýྻ�IJ�˿պȡԭ��Һ�ھƾ��ƻ��������գ��۲쵽��ɫ���档�����ƶ���ȷ���� �� ��
| A���϶���Na+��Al3+��Mg2+��SO42�� | B���϶���Na+��Mg2+��Al3+��HCO3�� |
| C���϶�û��K+��HCO3����MnO4�� | D���϶�û��K+��NH4+��Cl�� |
A
�����������ɫ��Һ�϶�û��MnO4������ȡ������Һ����������Na2O2���壬�����������Ǻ�ˮ��Ӧ�����������ƺ�������������ɫ��ζ��������������һ�����ǰ�������ʱ��ɫ�������֣��ټ���������NaOH��Һ���ɫ���������ܽ⣬������ijɷ���������þ��������������֤������һ������þ���Ӻ������ӣ�һ��������笠����ӡ�̼��������ӣ��������Ӳ����棩����ȡ������Һ������HNO3�ữ��Ba(NO3)2��Һ���а�ɫ��������������������ӷ�Ӧ���ɰ�ɫ����������İ�ɫ���������ᱵ������֤��һ��������������ӡ���ѡ��Aѡ�

��ϰ��ϵ�д�
 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�
�����Ŀ
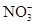 ��SCN��
��SCN��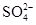
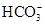
 HCO3����H��
HCO3����H��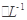 C6H5OH��Һ��ˮ�ĵ���̶�
C6H5OH��Һ��ˮ�ĵ���̶�