��Ŀ����
����Ŀ��75��Ԫ���Re���۵�������٣������캽�շ������ı���Ԫ�ء��ؿ���蝹ĺ������ͣ���������⡢ͭ��п��Ǧ�ȿ����С��ش��������⣺
��1����ԭ�Ӽ۲���ӵĹ����ʾʽΪ__���̴������ڱ���__����
��2����練�����ͭ���γɶ��������磺���������ͭ(I)[Cu(NH3)2]Ac���������պϳɰ��жԴ����к���CO���壺[Cu(NH3)2]Ac+CO+NH3![]() [Cu(NH3)3]Ac��CO��(Ac��ʾ�����)
[Cu(NH3)3]Ac��CO��(Ac��ʾ�����)
����ͭ�����γ������ķ��ӻ�����Ӧ�߱��Ľṹ������__��
�ڴ������Cԭ�ӵ��ӻ�����Ϊ__��1mol������[Cu(NH3)2]+�к�����������ĿΪ__��
��д����NH3��Ϊ�ȵ������һ�����ӵĻ�ѧʽ__��
��3������華���ͬһ�壬����蝹��۵�����̣�ԭ����___��
��4���������Ϊ������������������Ϊapm��������蝹�Ħ������ΪMg/mol���ԭ��ռ�ݶ��㣬��ԭ��ռ���������ġ����ԭ�ӵ���λ��Ϊ__���ԭ����������ԭ��Χ�ɵĿ�϶��___����������������������������������������������蝹��ܶ�Ϊ__g/cm3��(��NA��ʾ�����ӵ�������ֵ)
���𰸡�![]() d ���й¶Ե���(��¶Ե���) sp3��sp2 8NA H3O+��CH3�� ��еĽ�����ǿ���� 6 ������
d ���й¶Ե���(��¶Ե���) sp3��sp2 8NA H3O+��CH3�� ��еĽ�����ǿ���� 6 ������ ![]()
��������
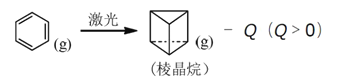
����Ԫ����25��Ԫ�أ���ԭ�Ӽ۵���Ϊ3d74s2��
�Ƣ�ͭ�����пչ���������ͭ�����γ������ķ��ӻ�����Ӧ���йµ��Ӷԣ��ڴ����������̼ԭ�ӵļ۵��Ӷ����ֱ�Ϊ4����3����[Cu(NH3)2]+��ÿ��ͭ�����γ�2����λ����ÿ�������������3�����ۼ����۸��ݼ۵���C�� = N = O+��������д����NH3��Ϊ�ȵ���������ӡ�
���̺�蝹ľ��嶼���ڽ������壬���۵�������������
���������Ϊ�����������ԭ��ռ�ݶ��㣬��ԭ��ռ���������ģ���ÿ����������1���ԭ�Ӻ�3����ԭ�ӣ�ÿ���ԭ�ӵ��ϡ��¡����ҡ�ǰ������һ���Ⱦ����ԭ�ӣ����ݹ�ʽ���������蝹��ܶȡ�
����Ԫ����25��Ԫ�أ���ԭ�Ӽ۵���Ϊ3d74s2���۵����Ų�ͼΪ![]() ��������Ԫ�����ڱ�d�����ʴ�Ϊ��
��������Ԫ�����ڱ�d�����ʴ�Ϊ��![]() ��d��
��d��
�Ƣ�ͭ�����пչ���������ͭ�����γ������ķ��ӻ�����Ӧ���йµ��Ӷԣ��ʴ�Ϊ�����й¶Ե���(��¶Ե���)��
�ڴ����������̼ԭ�ӵļ۵��Ӷ����ֱ�Ϊ4����3���������ӻ��������Ϊsp3��sp2��[Cu(NH3)2]+��ÿ��ͭ�����γ�2����λ����ÿ�������������3�����ۼ������1mol[Cu(NH3)2]+�������к�����������ĿΪ8NA���ʴ�Ϊ��sp3��sp2��8NA��
�۸��ݼ۵���C�� = N = O+����NH3��Ϊ�ȵ������������H3O+��CH3�����ʴ�Ϊ��H3O+��CH3����
���̺�蝹ľ��嶼���ڽ������壬���۵�����������������蝹��۵�����̣�˵���̵Ľ���������画��ʴ�Ϊ����еĽ�����ǿ���̡�
���������Ϊ������������������Ϊapm���ԭ��ռ�ݶ��㣬��ԭ��ռ���������ģ���ÿ����������1���ԭ�Ӻ�3����ԭ�ӣ�ÿ���ԭ�ӵ��ϡ��¡����ҡ�ǰ������һ���Ⱦ����ԭ�ӣ����ԭ�ӵ���λ��Ϊ6���ԭ����������ԭ��Χ�ɵİ������϶�У�������蝹��ܶ�Ϊ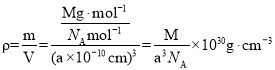 ���ʴ�Ϊ��6�������壻
���ʴ�Ϊ��6�������壻![]() ��
��

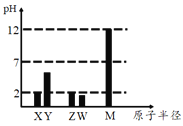
����Ŀ����ΪԪ�����ڱ���һ���֣�����a��f�������ֶ���������Ԫ�ء����������գ�
a | b | c |
d | e | f |
��1������Ԫ���У�ԭ�Ӱ뾶������_________ (��Ԫ�ر��) ��d��e��f����Ԫ�ص�ԭ�ӽṹ�ϵ���ͬ����________________________________��
��2����a����̬�⻯���ˮ��Һ�ʼ��ԣ���a����̬�⻯��ĵ���ʽ��___________������Ԫ���У�����������Ӧˮ�����������ǿ����_________(��Ԫ�ط���)��
��3����fԪ�ص�ԭ��L���������M���������1������eԪ�صķǽ����Ա�fԪ�صķǽ�����_________(ѡ����ǿ������������)����˵����һ��ʵ�Ļ�ѧ����ʽ��____________________(��дһ��)��
��4����bΪ�ǽ���Ԫ�أ��������ƶ���ȷ����_________(ѡ����)��
��aһ���ǽ���Ԫ�� ��dһ���ǽ���Ԫ�� ��fһ���Ƿǽ���Ԫ��
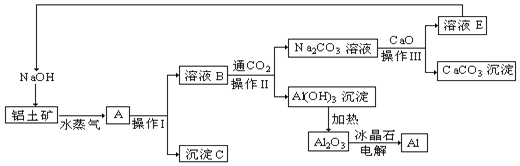
����Ŀ��ijʵ��С��������ͼװ�ã��ⶨij�־����Ͻ𣨺�Al2O3������������NaOH��Һ�ijɷ֣���Al����������������100mLNaOH��Һ������ÿ����ͬʱ����õ�����ƽ�����������
�������� | �������� | ����(g) |
��Ʒ | ��1�� | 2.582 |
��ƿ+100mL NaOH��Һ | ��2�� | 185.721 |
��ƿ+NaOH��Һ+��Ʒ | ��3�� | 188. 286 |
��4�� | 188. 254 | |
��5�� | 188. 243 | |
��6�� | 188. 243 |
��1����Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________��__________________________________________________��
��2����Ӧ�в�����H2����Ϊ___________________ g��
��3����Ʒ��Al����������Ϊ___________________����С����ʾ��С���������λ������С��Ϊ�˲ⶨ����Al2O3�ĺ����������淴Ӧ���100mL��Һ�У�ȡ��10mL��Һ����μ���1mol/L�����ᣬ���ε�5.00mLʱ��ʼ���������������μ����ᣬ��25.00mLʱ����ǡ��ȫ���ܽ⡣
��4��ȡ����10mL��Һ�У�AlO2�������ʵ���Ũ��Ϊ_____________________________��
��5��Al2O3����������_____________________����С����ʾ��С���������λ����