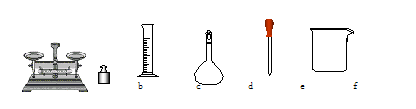��Ŀ����
��8�֣�ijͬѧ��10mol/L��Ũ��������250mL 1mol/L��ϡ���ᣬ�������й�ʵ�顣��ش��������⣺
��1����Ҫ��ȡŨ����___________mL��
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ�����������250 mL����ƿ�⣬�������õ���������________�ȡ�
��3��ȡ�����Ƶ�ϡ����100mL����һ��������п��ַ�Ӧ��пȫ���ܽ�����ɵ������ڱ�״���µ����Ϊ0.896L���跴Ӧ����Һ�������Ϊ100mL����Ӧ����Һ��H+�����ʵ���Ũ��Ϊ______________��
��4����һƿ��������Ϊ14�G��KOH��Һ������������200g H2O�õ�160 mL��������Ϊ28�G��KOH��Һ����������Һ��KOH���ʵ���Ũ��Ϊ______________��
��1����Ҫ��ȡŨ����___________mL��
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ�����������250 mL����ƿ�⣬�������õ���������________�ȡ�
��3��ȡ�����Ƶ�ϡ����100mL����һ��������п��ַ�Ӧ��пȫ���ܽ�����ɵ������ڱ�״���µ����Ϊ0.896L���跴Ӧ����Һ�������Ϊ100mL����Ӧ����Һ��H+�����ʵ���Ũ��Ϊ______________��
��4����һƿ��������Ϊ14�G��KOH��Һ������������200g H2O�õ�160 mL��������Ϊ28�G��KOH��Һ����������Һ��KOH���ʵ���Ũ��Ϊ______________��
��1��25 ��2����ͷ�ι� ��3��0.2mol/L ��4��6.25 mol/L
��1������ҪŨ��������ΪVL��VL��10mol/L=0.25L��1mol/L V=0.025L=25ml
����Ҫ��ȡŨ����25ml
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ�����������250 mL����ƿ�⣬�������õ��������н�ͷ�ιܡ�
��3��Zn��2HCl=ZnCl2��H2�� ���ɵ������ڱ�״���µ����Ϊ0.896L��Ϊ0.04mol����ȡ100ml�����к��Ȼ���0.1mol����Ӧ��0.08mol���ᣬ��ʣ��0.02mol�����ԣ���Һ��H+�����ʵ���Ũ��Ϊ0.02mol/0.1L=0.2mol/L
��4����ԭ��Һ����ΪXg����KOH����Ϊ0.14Xg����������Һ����Ϊ��X��200��g����������ǰ�������������䣬�ɵ�0.14X/��X��200��=0.28�����X=400��ԭ��Һ����Ϊ400g
KOH����Ϊ400��0.14=56g�����ʵ���Ϊ1mol�����ʵ���Ũ��Ϊ��1/0.16��mol/L��Ϊ6.25
mol/L��
����Ҫ��ȡŨ����25ml
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ�����������250 mL����ƿ�⣬�������õ��������н�ͷ�ιܡ�
��3��Zn��2HCl=ZnCl2��H2�� ���ɵ������ڱ�״���µ����Ϊ0.896L��Ϊ0.04mol����ȡ100ml�����к��Ȼ���0.1mol����Ӧ��0.08mol���ᣬ��ʣ��0.02mol�����ԣ���Һ��H+�����ʵ���Ũ��Ϊ0.02mol/0.1L=0.2mol/L
��4����ԭ��Һ����ΪXg����KOH����Ϊ0.14Xg����������Һ����Ϊ��X��200��g����������ǰ�������������䣬�ɵ�0.14X/��X��200��=0.28�����X=400��ԭ��Һ����Ϊ400g
KOH����Ϊ400��0.14=56g�����ʵ���Ϊ1mol�����ʵ���Ũ��Ϊ��1/0.16��mol/L��Ϊ6.25
mol/L��

��ϰ��ϵ�д�
�����Ŀ