��Ŀ����
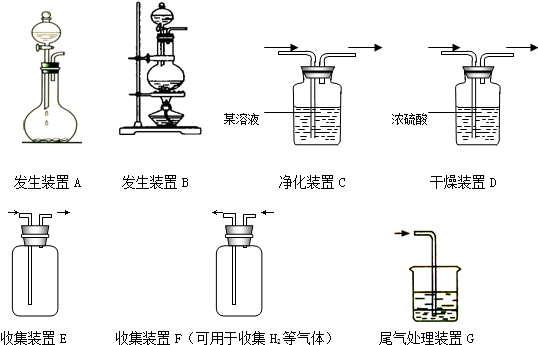
CO2�����Ǵ�����������ЧӦ��ʹ�����ů������֮һ��ijѧ���μӿ����С�飬�ⶨУ�칤���ų�������CO2��������SO2���ĺ�����������CO2��ɳ�����Ȼ������������ͨ���������CO2�ĺ�����ͼ�б�ʾ��Ҫװ�ã��������ܡ�������ȥ��

�����������Ҫ����װ��ʹ�õ�˳�������֣����� ������Ϊ������
������A��D��E��F��C �ҷ�����A��D��F��E��C
��ΪʲôҪʹ��������ͨ������KMnO4��Һ
��Ϊʲôû��ѡBװ��
��CO2�DZ�ת���� ������������������ġ�
��Ҫ��ó�������������� �� ����� ������
��1����
��2��ʹSO2�����ܱ���ȥ�� E����Һ����ɫ��ȷ��SO2����
��3��NaHCO3��SO2��Ӧ������CO2��ʹԭ��CO2�������������
��4��BaCO3
��5�����ˡ�ϴ�ӡ�������� ��ÿ��1�֣���7�֣�

��ϰ��ϵ�д�
�����Ŀ
 ����NO2��SO2��CO���к�����Դ���������Σ����������Ĺ�ͬ��
����NO2��SO2��CO���к�����Դ���������Σ����������Ĺ�ͬ��