��Ŀ����
20�����������ȵ�����ͭ��Ӧ����ͭ��������ˮ������ͼ1��ʾʵ��װ�ÿɲⶨ�����ӵ���ɣ�ͼ�мг֡��̶�װ�ò��־���ȥ�����ش��������⣺
��1��д������������ͭ��Ӧ�Ļ�ѧ����ʽ��2NH3+3CuO $\frac{\underline{\;\;��\;\;}}{\;}$N2+3Cu+3H2O��
��2����A����ƿ�з���NaOH�����Ŀ��������NaOH�ļ��Ժ���ˮ���ȵ����ʣ�ʹNH3•H2O�ֽ�����NH3���ݳ���
��3���ڸ����B�в���ѡ�õĸ������C������ţ���
A����ʯ�� B����ʯ�� C������������ D����������
��4��Eװ����ʢװŨ�����Ŀ���ǣ����ն����NH3����ֹF�е�ˮ��������D�У�
��5����ʵ����Ϻ���ʵ����N2�����������ɱ�״����a L�������D����b g�������е������ԭ�Ӹ�����Ϊ���ú�a��b��ĸ�Ĵ���ʽ��ʾ��$\frac{9a}{11.2b}$��
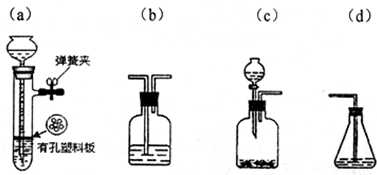
��6��ʵ���ҿ���ͼ2��ʾװ�ã�ȱ���ռ�װ�ã��г̶ֹ�װ����ȥ���Ʊ����ռ�NH3
����ͼ2�з����ڻ�������ƿ�ռ�������װ�ü�ͼ��
���ձ�����Һ����ɫ��Ϊ��ɫ����ԭ���ǣ��õ��뷽��ʽ��ʾ��NH3•H2O?NH4++OH-��
���� ��1�����������ȵ�����ͭ��Ӧ����ͭ��������ˮ�����������غ���д��Ӧ�Ļ�ѧ����ʽ��
��2���������������ܹ���������������ӡ�������������ˮ�ų������ȷ��������
��3������Ϊ�������壬���������Ը�������
��4��Ũ�����ܹ�����δ��Ӧ�İ��������ܹ���ֹF��ˮ�ֽ���D��
��5������n=$\frac{V}{Vm}$��������������ʵ������ٸ��ݷ�Ӧ����ʽ2NH3+3CuO $\frac{\underline{\;\;��\;\;}}{\;}$N2+3Cu+3H2O���������ԭ������ͭ�����ʵ���������n=$\frac{m}{n}$�������Ӧ����ˮ�����ʵ������ٸ��������غ㶨�ɼ����N��Hԭ����֮�ȣ�
��6���ٰ������ܶ�С�ڿ�����������ˮ�������ռ��������ſ������ռ���
�ڰ�������ˮ�Լ��ԣ���ʹ��̪�Ժ�ɫ���ݴ˴��⣮
��� �⣺��1�����������ȵ�����ͭ��Ӧ����ͭ��������ˮ����Ӧ�Ļ�ѧ����ʽΪ��2NH3+3CuO $\frac{\underline{\;\;��\;\;}}{\;}$N2+3Cu+3H2O��
�ʴ�Ϊ��2NH3+3CuO $\frac{\underline{\;\;��\;\;}}{\;}$N2+3Cu+3H2O��
��2��Ũ��ˮ�д���ƽ�⣺NH3+H2O?NH3•H2O?NH4++OH-��������������Һ�е�������������ӣ������ڰ��������ɣ���������������ˮ�ų������������˰������ܽ�ȣ�
�ʴ�Ϊ������NaOH�ļ��Ժ���ˮ���ȵ����ʣ�ʹNH3•H2O�ֽ�����NH3���ݳ���
��3�������������ܹ��백����Ӧ�����ܸ��ﰱ������ѡC��
��4��Ũ�����ܹ�����Ӧʣ��İ����գ���ֹF�е�ˮ��������D�У����������
�ʴ�Ϊ�����ն����NH3����ֹF�е�ˮ��������D�У�
��5����״���£�aL���������ʵ���Ϊ��n��N2��=$\frac{aL}{22.4L/mol}$=$\frac{a}{22.4}$mol�����ݷ�Ӧ2NH3+3CuO $\frac{\underline{\;\;��\;\;}}{\;}$N2+3Cu+3H2O��֪������ԭ��CuO�����ʵ���Ϊ��n��CuO��=3n��N2��=$\frac{a}{22.4}$mol��3=$\frac{3a}{22.4}$mol��
װ��D���ص�Ϊˮ����������ˮ�����ʵ���Ϊ��$\frac{bg}{18g/mol}$=$\frac{b}{18}$mol�����е�H�����ʵ���Ϊ��$\frac{b}{18}$mol��2=$\frac{b}{9}$mol��
�����к��е�N��Hԭ����֮��Ϊ���� $\frac{a}{22.4}$mol��2����$\frac{b}{9}$mol=$\frac{9a}{11.2b}$��
�ʴ�Ϊ��$\frac{9a}{11.2b}$��
��6���ٰ������ܶ�С�ڿ�����������ˮ�������ռ��������ſ������ռ���װ����ͼ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
�ڰ�������ˮ�Լ��ԣ����뷽��ʽΪNH3•H2O?NH4++OH-����ʹ��̪�Ժ�ɫ���ʴ�Ϊ��NH3•H2O?NH4++OH-��
���� ���⿼���˰������Ʒ�����ѧ���ʣ���Ŀ�Ѷ��еȣ�ע��������ȡ�����ķ�Ӧԭ���������Ļ�ѧ���ʣ���ȷʵ��ⶨԭ��Ϊ�����Ĺؼ�����5��Ϊ�״��㣬��Ҫ��ȷװ��BD�����ã�

| ѡ �� | ���������� | �� Һ |
| A | ����NaOH����Һ������ɫ�������ټ��������ǣ����ȣ�������ɫ������ | CuSO4��Һ |
| B | ����NaOH����Һ������ɫ��������ͨ��CO2����ɫ������ʧ�� | Ca��HCO3��2��Һ |
| C | ����NaOH����Һ��ɫ��ȥ���ټ������ᣬ����Һ�ֱ����ɫ�� | I2��CCl4��Һ |
| D | ����NaOH����Һ������ɫ�������д̼�����ζ����������������� NaOH��������ʧ�� | KAl��SO4��2��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | һ��С��92.0 kJ | B�� | һ������92.0 kJ | C�� | һ������92.0 kJ | D�� | ����ȷ�� |
| A�� | ����������ȼ�ջ���ʰ�ɫ�����ɵ���ɫ���� | |
| B�� | ����ϡ���ᷴӦ���������������壬�����ػ�ɫ��Һ | |
| C�� | ͭ��������Ӧ�����ػ�ɫ���̣�������ˮ����Һ������ɫ | |
| D�� | ������������ȼ�ղ�����ɫ�����������д̼�����ζ������ |
| A�� | �ߴ��ȵĹ赥�ʹ㷺�����������ά�����ά��ǿ��ᡰ��·�� | |
| B�� | �����Ƽ�Ĺ���ԭ����ҪӦ���������۷е�IJ��� | |
| C�� | ���Ͻ�Ĵ���ʹ�ù鹦��������ʹ�ý�̿�Ȼ�ԭ�����������л���� | |
| D�� | ��պ��Ũ��ˮ�IJ������������������Ĺܵ��Ƿ�©�� |
| A�� | ��AΪO2��BΪO3�������������ʱ����������ԭ�Ӹ�������� | |
| B�� | ��AΪNO2��BΪN2O4������ѹǿ���ʱ��������� | |
| C�� | ��AΪC2H4��BΪC2H6�������ܶ����ʱ����������ʵ������ | |
| D�� | ��AΪCO2��BΪC3H8�������ܶ����ʱ���������� |
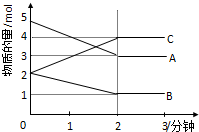 ij���淴Ӧ��0-2���ӽ��й����У��ڲ�ͬ��Ӧʱ������ʵ����ı仯�����ͼ��ʾ��
ij���淴Ӧ��0-2���ӽ��й����У��ڲ�ͬ��Ӧʱ������ʵ����ı仯�����ͼ��ʾ��