��Ŀ����
����Ŀ�����ᡢ�����������г��������ʡ�
��1��25��ʱ��Ũ�Ⱦ�Ϊ0.1mol��L-1������ʹ�����Һ������˵����ȷ����___��
a.����Һ��pH��ͬ
b.����Һ�ĵ���������ͬ
c.����Һ����ˮ�������c(OH-)��ͬ
d.�к͵����ʵ�����NaOH����������Һ�������ͬ
��2��25��ʱ��pH������4�Ĵ�����Һ��������Һ��������Һ��ˮ�������H��Ũ����������Һ��ˮ�������H��Ũ��֮����___��
��3��������Һ�д��ڵ���ƽ�⣺CH3COOH![]() CH3COO-��H����������������ȷ����___��
CH3COO-��H����������������ȷ����___��
a.CH3COOH��Һ������Ũ�ȹ�ϵ���㣺c(H��)=c(OH-)��c(CH3COO)
b.0.1mol��L-1��CH3COOH��Һ��ˮϡ�ͣ���Һ��c(OH��)��С
c.CH3COOH��Һ�м�������CH3COONa���壬ƽ�������ƶ�
d.�����£�pH=2��CH3COOH��Һ��pH=12��NaOH��Һ�������Ϻ���Һ��pH��7
e.������pH=3�Ĵ�����Һ��ˮϡ�ͣ���Һ��![]() ����
����
��4�������£���pH��ͬ�������ͬ�Ĵ��������������Һ����ȡ���´�ʩ��
a.��ˮϡ��10��������Һ�е�c(H��)__(����>����=������<��)������Һ�е�c(H��)��
b.�ӵ�Ũ�ȵ�NaOH��Һ��ǡ���кͣ�����NaOH��Һ�����������__(����>����=������<��)���ᡣ
c.ʹ�¶ȶ�����20�棬��Һ��c(H��)������__(����>����=������<��)���ᡣ
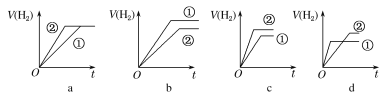
d.�ֱ���������п�۷�����Ӧ�����й����������(V)��ʱ��(t)�仯��ʾ��ͼ��ȷ����__(����ĸ)��(�ٱ�ʾ���ᣬ�ڱ�ʾ����)
��5����0.1mol��L-1��CH3COOH��ˮϡ�ͣ��й�ϡ�ͺ������Һ��˵���У���ȷ����__(����ĸ)��
a.����̶�����
b.��Һ��������������
c.��Һ��������ǿ
d.��Һ�д����������
���𰸡�d 1��1 bd > > > c ab
��������
(1)a��CH3COOH��������ʣ�HCl��ǿ����ʣ���Ũ�ȵ�������Һ��������c(H+)����CH3COOH�����Դ����pH����HCl���ʴ���
b��CH3COOH��������ʣ�HCl��ǿ����ʣ���Ũ�ȵ�������Һ������������Ũ�ȴ��ڴ��ᣬ��Һ�ĵ�������������Ũ�ȳ����ȣ�����HCl��Һ�ĵ�������ǿ���ʴ���
c��������ˮ���룬����������Ũ��Խ�������Ƴ̶�Խ��Ũ�ȵ��������У�HCl��������Ũ�ȴ��ڴ��ᣬ������ˮ�������c(OH-)����������ᣬ�ʴ���
d���к͵����ʵ�����NaOH��Һ�����������������Ũ�ȳɷ��ȣ�����������ʵ���Ũ����ȣ�������Ҫ��������ȣ�����ȷ��
��ѡ�ܣ�
(2)25��ʱ��pH������4�Ĵ�����Һ��������Һ������������c(H+)��ȣ����ˮ�ĵ������Ƴ̶���ͬ�����Դ�����Һ��ˮ�������H��Ũ����������Һ��ˮ�������H��Ũ��֮����1:1��
(3)a��CH3COOH��Һ�У����ݵ���غ��֪��c(H+)=c(OH-)+c(CH3COO)����a��ȷ��
b��0.1mol/L��CH3COOH��Һ��ˮϡ�ͣ���Һ���Լ���������Һ��c(OH-)����b����
c��CH3COOH��Һ�м�������CH3COONa���壬���������Ũ��������ƽ�������ƶ�����c��ȷ��
d�������£�pH=2��CH3COOH��ҺŨ�ȴ���pH=12��NaOH��Һ���������Ϻ������������Һ�����ԣ���Һ��pH��7����d����
e���������ˮ��ƽ�ⳣ��Kh=![]() ���¶Ȳ���ˮ��ƽ�ⳣ�����䣬����������pH=3�Ĵ�����Һ��ˮϡ�ͣ���Һ��
���¶Ȳ���ˮ��ƽ�ⳣ�����䣬����������pH=3�Ĵ�����Һ��ˮϡ�ͣ���Һ��![]() ���䣬��e��ȷ��
���䣬��e��ȷ��
��������ѡbd��
(4)a��pH��ͬ�������ͬ�Ĵ��������������Һ�ֱ��ˮϡ�ͺ���Һ��������Ũ�ȶ���С������pH����������������ڵ���ƽ�⣬�ֵ���������ӣ�������Һ�е�c(H+)��������Һ�е�c(H+)��
b��pH��ͬ�Ĵ�������ᣬ�����Ũ�ȴ������ᣬ�ӵ�Ũ�ȵ�NaOH��Һ��ǡ��ǡ���кͣ�����NaOH��Һ�������������
c��������ǿ�ᣬ�����ڵ���ƽ�⣬�����¶Ȳ�Ӱ�������pH�����������ᣬ��ˮ��Һ�д��ڵ���ƽ�⣬�����¶ȣ��ٽ�������룬���´�����Һ��������Ũ������������Һ��c(H+)��������
d����Ϊǿ����ȫ���룬һԪ���Ჿ�ֵ��룬���Ҫ����ͬ��pHֵ��һԪ�����Ũ�ȱ����ǿ������������ȣ����һԪ��������ʵ�������ǿ�ᣬ��˲�����H2Ҳ��ǿ��࣬�ݴ˿��ų�a��b����ʼʱH+Ũ����ȣ���˷�Ӧ����Ҳ���(��ͼ�з�Ӧ���ʾ���б��)�����ŷ�Ӧ��������H+���ϱ����ĵ�����ʹһԪ����������룬����ӻ���H+Ũ���½������ʣ���ʱ����ķ�Ӧ���ʾ�Ҫ����ǿ��ķ�Ӧ���ʣ�����������H2�����һԪ�����ѡc��
(5)��0.1molL-1��CH3COOH��ˮϡ�ͣ���ҺŨ�ȼ�С�����������ĵ���ƽ�����ƣ�
a����ˮ����ĵ���ƽ�����ƣ��ʵ���̶�����a��ȷ��
b����ˮ����ƽ�����ƣ�����Һ�������������࣬��b��ȷ��
c����ˮ����Ȼ����������ӵ����ʵ������࣬����Һ�������Ũ�ȼ�С������Һ�����Լ�������c����
d����ˮ����ƽ�����ƣ�����Һ�д�����Ӽ��٣���d����
��ѡab��

 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�