��Ŀ����
12����ҵ���������β���к��е�������NOx��NO��NO2�Ļ������費��N2O4��������̬���������ཡ�������ϴ����в����1����ҵ�Ͽ��ð������շ�����NOx����Ӧԭ�����£�4xNH3+6NOx$\frac{\underline{\;����\;}}{\;}$��2x+3��N2+6xH2Oij��ѧ��ȤС��ģ��ô������̵�ʵ��װ����ͼ1��
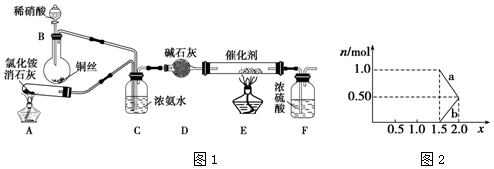
��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��װ��D�м�ʯ�ҵ������dz�ȥ�����к��е�ˮ������
��2����ҵ��Ҳ����Na2CO3��Һ���շ�����NOx��
��֪��NO������Na2CO3��Һ��Ӧ��
NO+NO2+Na2CO3�T2NaNO2+CO2����
2NO2+Na2CO3�TNaNO2+NaNO3+CO2����
�ٵ�NOx��Na2CO3��Һ��ȫ����ʱ��x��ֵ��������C������ĸ����
A��1.9 B��1.7 C��1.2
�ڽ�1mol NOxͨ��Na2CO3��Һ�У�����ȫ����ʱ����Һ�����ɵ�NO3-��NO2-�������ӵ����ʵ�����x�仯��ϵ��ͼ2��ʾ��
ͼ���߶�a��ʾNO2-�����ʵ�����xֵ�仯�Ĺ�ϵ������������������Ϊ42.4%�� Na2CO3��Һ���գ�����ҪNa2CO3��Һ����125g��
����������Na2CO3��Һ��ȫ����NOx��ÿ����22.4L����״����CO2��ȫ���ݳ���ʱ������Һ����������44g����NOx�е�xֵΪ1.875��
���� ��1�����ڼ��������£��Ȼ�狀��������Ʒ�Ӧ�����Ȼ��ơ�������ˮ��
�ڰ������ڼ������壬���ü������ʸ��
��2���ٵ�NOx��Na2CO3��Һ��ȫ����ʱ����n��NO2����n��NO����
�����ü����ͷ�Ӧ���ɵ�NaNO3��NaNO2�е�Ԫ������Ԫ��֮��Ϊ1��1���������
�����ò���������NO��NO2�����ʵ������ٸ���ƽ��Ħ������������xֵ��
��� �⣺��1�����ڼ��������£��Ȼ�狀��������Ʒ�Ӧ�����Ȼ��ơ�������ˮ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ڼ�ʯ��������ˮ�ֶ�����������������ڼ������壬�������ü�ʯ�Ҹ��
�ʴ�Ϊ����ȥ�����к��е�ˮ������
��2���ٵ�NOx��Na2CO3��Һ��ȫ����ʱ����n��NO2����n��NO������n��NO2����n��NO��=1ʱxֵ��С��x��СֵΪ$\frac{2+1}{2}$=1.5����Ϊ����NO������x���ֵ��2����x��ȡֵ��ΧΪ1.5��x��2������x��ֵ��������1.2��
�ʴ�Ϊ��C��
���ü�������x=1.5����ӦΪNO��NO2�������ʵ�����Ϊ1��1������ʽ��Ӧ��û��NO3-����aӦ�ñ�ʾNO2-��
���غ㷨����Ӧ���ɵ�NaNO3��NaNO2�е�Ԫ������Ԫ��֮��Ϊ1��1������1mol NOx����ȫ����������̼����0.5mol������Ϊ53g������������������������Ϊ42.4%�� Na2CO3��Һ����$\frac{53}{42.4%}$=125g��
�ʴ�Ϊ��NO2-��125��
������NO2�ʹ��Ӧ����CO2Ϊamol��
��NO��NO2�봿�Ӧ������CO2Ϊbmol��
2NO2+Na2CO3=NaNO2+NaNO3+CO2 ��������
1mol��m=48g
amol 48ag
NO+NO2+Na2CO3=2NaNO2+CO2 ��������
1mol��m=32g
bmol 32bg
$\left\{\begin{array}{l}{a+b=1}\\{48a+32b=44}\end{array}\right.$��
���a=0.75mol��b=0.25mol��
n��NO2��=0.75mol��2+0.25mol=1.75mol
n��NO��=0.25mol
x=$\frac{0.25mol��1+1.75mol��2}{0.25mol+1.75mol}$=1.875��
�ʴ�Ϊ��1.875��
���� �����Ե�������Ϊ���忼�������ʼ�ķ�Ӧ����ȷ���ʵ������ǽⱾ��ؼ����ѵ��ǣ�2����ļ��㣬���շ�Ӧ�����ʵ�����ϵ��ԭ���غ㡢������Ϊ���Ĺؼ������ط�������������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�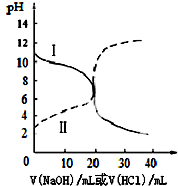 25��ʱ��ȡŨ�Ⱦ�Ϊ0.1mol•L-1�Ĵ�����Һ�Ͱ�ˮ��Һ��20mL���ֱ���Ũ�Ⱦ�Ϊ0.1mol•L-1NaOH��Һ����������к͵ζ����ζ�������pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
25��ʱ��ȡŨ�Ⱦ�Ϊ0.1mol•L-1�Ĵ�����Һ�Ͱ�ˮ��Һ��20mL���ֱ���Ũ�Ⱦ�Ϊ0.1mol•L-1NaOH��Һ����������к͵ζ����ζ�������pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | ���ߢμ���Һ��20 mLʱ��Һ��pH=5��c��H+��-c����NH3•H2O��=c��OH-��=1��10-9 mol•L-1 | |
| B�� | ���ߢμ���Һ��20 mLʱ��c��Cl-����c��NH4+����c��H+����c��OH-�� | |
| C�� | ���ߢμ���Һ��10 mL��20 mL֮����ڣ�c��NH4+��=c��Cl-����c��OH-��=c��H+�� | |
| D�� | ���ߢμ���Һ��10 mLʱ��c��CH3COO-��-c��CH3COOH��=2[c��H+��-c��OH-��] |
| A�� | ��������������ﶼ�����壬ͨ��ˮ�ж��γ�ǿ�� | |
| B�� | ��FeCl3���뱥��H2S��Һ�У���Һ��pH���� | |
| C�� | ��CO2ͨ��CaSO4��Һ�У����������� | |
| D�� | ������ú������ȿ�ʵ����Դ����Ч���ã����ɼ���������Ⱦ |
| A�� | 5.6g���������ᷴӦʧȥ�ĵ�����һ��Ϊ0.3��6.02��1023 | |
| B�� | 2.24 L CO2�к��е�ԭ����Ϊ0.3��6.02��1023 | |
| C�� | 200 mL 5 mol•L-1 Na2CO3��Һ�У���6.02��1023��CO32- | |
| D�� | 4.5gSiO2�����к��еĹ�������ĿΪ0.3��6.02��1023 |
| A�� | �̷�һFeSO4•7H2O | B�� | â��һNa2SO4•10H2O | ||
| C�� | ����һAl��SO4��3•12H2O | D�� | ����һCuSO4•5H2O |
| A�� | ����������Һ | B�� | ʳ��ˮ | C�� | Ũ���� | D�� | ���� |
| A�� | ��������ˮ��Cl2+H2O�T2H++Cl-+ClO- | |
| B�� | Ba��OH��2��Һ��H2SO4��Һ��Ӧ��Ba2++2H++2OH-+SO42-�TBaSO4��+2H2O | |
| C�� | Na2CO3��Һ��ϡ���ᷴӦ��CO32-+2H+�TCO2��+H2O | |
| D�� | NH4Cl ��Һ�����ԣ�NH4++H2O?NH3•H2O+H+ |
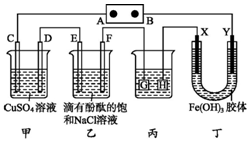 ��ͼװ����ʾ��C��D��E��F��X��Y���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ������ͨ��ǰ����Һ������䣩��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ��F�������ʺ�ɫ��
��ͼװ����ʾ��C��D��E��F��X��Y���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ������ͨ��ǰ����Һ������䣩��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ��F�������ʺ�ɫ��