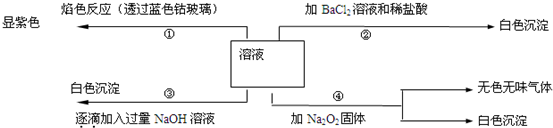
��Ŀ����
��һ��ɫ��Һ�����п��ܺ���Fe3+��Al3+��Mg2+��Cu2+��NH4+��K+��CO32-��SO42-�������еļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й�������ͼ��ʾ��
�ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH����������ͼ��ʾ�����ϵ��
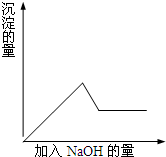
�ݴ˿�֪����1����ԭ��Һ��һ�����ڵ�������______��
��2��д���ڢ۸�ʵ���з�����Ӧ�����ӷ���ʽ______��
��3��д���ڢܸ�ʵ������������Ļ�ѧ����ʽ______��

�ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH����������ͼ��ʾ�����ϵ��

�ݴ˿�֪����1����ԭ��Һ��һ�����ڵ�������______��
��2��д���ڢ۸�ʵ���з�����Ӧ�����ӷ���ʽ______��
��3��д���ڢܸ�ʵ������������Ļ�ѧ����ʽ______��
��1����Һ����ɫ�ܲ�����ɫ��Ӧ����ɫ��˵����Һ�к���K+���ӣ���Һ����ɫ����һ������Fe3+���ӣ�
��μ������NaOH��Һ�а�ɫ����������Һ�к���Mg2+���ӣ�
�����Ȼ�������������ɰ�ɫ������������Һ��û��Ag+���ӣ������ɳ���ΪBaSO4����Һ�к���SO42-���ӣ�
�ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������������С����˵����Һ�к���Al3+���ӣ�
��ԭ��Һ��һ������Al3+��Mg2+��K+��SO42-�����ӣ�
�ʴ�Ϊ��Al3+��Mg2+��K+��SO42-��
��2����Һ�к���Al3+��Mg2+������NaOHʱ�ȷ�����ӦΪ��Mg2++2OH-=Mg��OH��2����Al3++3OH-=Al��OH��3��������Mg��OH��2��Al��OH��3��������ȫ�����ɳ���֮���ټ���NaOH������������NaOH������Ӧ����Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Mg2++2OH-=Mg��OH��2����Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O��
��3������������ˮ��Ӧ����NaOH����������Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2��
��μ������NaOH��Һ�а�ɫ����������Һ�к���Mg2+���ӣ�
�����Ȼ�������������ɰ�ɫ������������Һ��û��Ag+���ӣ������ɳ���ΪBaSO4����Һ�к���SO42-���ӣ�
�ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������������С����˵����Һ�к���Al3+���ӣ�
��ԭ��Һ��һ������Al3+��Mg2+��K+��SO42-�����ӣ�
�ʴ�Ϊ��Al3+��Mg2+��K+��SO42-��
��2����Һ�к���Al3+��Mg2+������NaOHʱ�ȷ�����ӦΪ��Mg2++2OH-=Mg��OH��2����Al3++3OH-=Al��OH��3��������Mg��OH��2��Al��OH��3��������ȫ�����ɳ���֮���ټ���NaOH������������NaOH������Ӧ����Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Mg2++2OH-=Mg��OH��2����Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O��
��3������������ˮ��Ӧ����NaOH����������Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2��

��ϰ��ϵ�д�
�����Ŀ