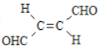��Ŀ����
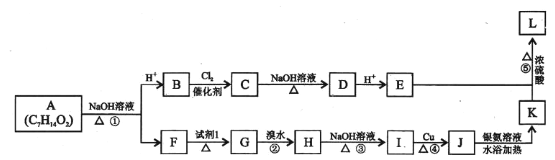
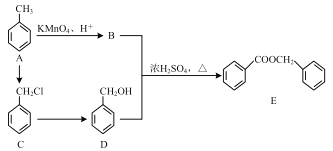
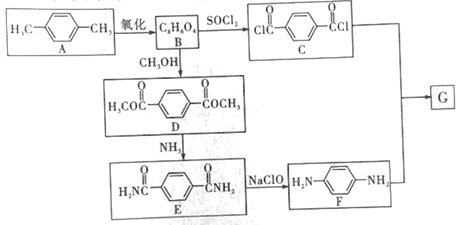
����Ŀ���й���ѧ�����ô�ɽ����Ƭ�������������������һָ��Եķ����£�����������湦�ܵ��Ǿ۶Ա��������Ա�����(G)����ϳ�·�����£�
�ش��������⣺
��1��A�Ļ�ѧ����Ϊ___��
��2��B�к��еĹ���������Ϊ___��B��C�ķ�Ӧ����Ϊ___��
��3��B��D�Ļ�ѧ��Ӧ����ʽΪ___��
��4��G�Ľṹ��ʽΪ___��
��5�����㻯����H��B��ͬ���칹�壬��������������H�Ľṹ����___��(�����������칹)�����к˴Ź��������������Ľṹ��ʽΪ___��
������NaHCO3��Һ��Ӧ����CO2�����ܷ���������Ӧ
��6�����������ϳ�·�ߣ������![]() Ϊԭ��(�����Լ���ѡ)���Ʊ�
Ϊԭ��(�����Լ���ѡ)���Ʊ�![]() �ĺϳ�·�ߣ�___��
�ĺϳ�·�ߣ�___��
���𰸡��Զ��ױ�����1��4-���ױ��� �Ȼ� ȡ����Ӧ ![]() +2CH3OH
+2CH3OH![]()
 +2H2O��
+2H2O�� 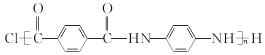 13
13 ![]()
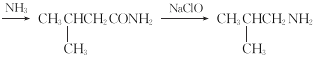
��������
���ݺϳ�·�߿�֪��![]() �������õ�B�����D�Ľṹ��ʽ�����Ƶõ�BΪ
�������õ�B�����D�Ľṹ��ʽ�����Ƶõ�BΪ![]() ��B��״�����������Ӧ�ɵõ�D��D�백������ȡ����Ӧ����E��E��������Ʒ�Ӧ�õ�F��F��C�������۷�Ӧ���ɾ۶Ա��������Ա�������G��������֪G�Ľṹ��ʽΪ��
��B��״�����������Ӧ�ɵõ�D��D�백������ȡ����Ӧ����E��E��������Ʒ�Ӧ�õ�F��F��C�������۷�Ӧ���ɾ۶Ա��������Ա�������G��������֪G�Ľṹ��ʽΪ��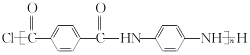 ������л���Ľṹ�����ʷ�������
������л���Ľṹ�����ʷ�������
��1������A�Ľṹ�����������ڶ�λ��������Ϊ�Զ��ױ�����ϵͳ����Ϊ1��4-���ױ���
�ʴ�Ϊ���Զ��ױ�����1��4-���ױ�����
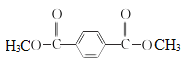
��2����D�Ľṹ���ƣ���֪BΪ�Ա������ᣨ![]() �������еĹ���������Ϊ�Ȼ����Ա�B��C�Ľṹ����֪B��C�ķ�Ӧ����Ϊȡ����Ӧ��
�������еĹ���������Ϊ�Ȼ����Ա�B��C�Ľṹ����֪B��C�ķ�Ӧ����Ϊȡ����Ӧ��
�ʴ�Ϊ���Ȼ���ȡ����Ӧ��
��3��B��DΪ������Ӧ����Ӧ�ķ���ʽΪ��![]() +2CH3OH
+2CH3OH![]()
 +2H2O��
+2H2O��
�ʴ�Ϊ��![]() +2CH3OH
+2CH3OH![]()
 +2H2O��
+2H2O��
��4����֪GΪ�۶Ա��������Ա���������֪C��F��һ�������·������۷�Ӧ���õ�����ṹ��ʽΪ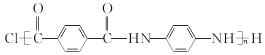 ��
��
�ʴ�Ϊ��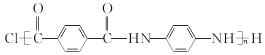
��4�����㻯����H��B��ͬ���칹�壬����![]() ��Һ��Ӧ����
��Һ��Ӧ����![]() ���ܷ���������Ӧ��˵�������Ȼ���ȩ�������ܵĽṹ��һ����������������ͬ�����ţ���CHO����COOH����OH����/span>10��ͬ���칹�壻������һ��������������ͬ�����ţ�HCOO������COOH�����ڡ��䡢�����֣���10+3=13�֡����к˴Ź���������������H�Ľṹ��ʽΪ
���ܷ���������Ӧ��˵�������Ȼ���ȩ�������ܵĽṹ��һ����������������ͬ�����ţ���CHO����COOH����OH����/span>10��ͬ���칹�壻������һ��������������ͬ�����ţ�HCOO������COOH�����ڡ��䡢�����֣���10+3=13�֡����к˴Ź���������������H�Ľṹ��ʽΪ![]() ��
��
�ʴ�Ϊ��13��![]() ��
��
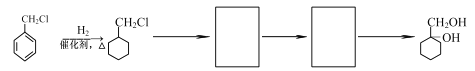
��6�������![]() Ϊԭ�ϣ��Ʊ�
Ϊԭ�ϣ��Ʊ�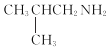 �ĺϳ�·�ߣ�������ĿB��D��D��E��E��F��Ϣ����ʵ�ְ��������뼰̼�������̣�����ĺϳ�·��Ϊ
�ĺϳ�·�ߣ�������ĿB��D��D��E��E��F��Ϣ����ʵ�ְ��������뼰̼�������̣�����ĺϳ�·��Ϊ
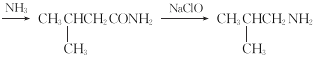 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�