��Ŀ����
�ں��¡����ݵ��ܱ������У��������A��B��C �����ʵ���Ũ��(c)��ʱ��(t) �Ĺ�ϵ���±���ʾ
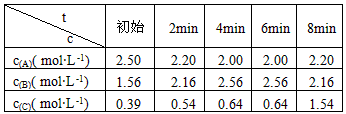
�����:
(1)ǰ2mim�ڣ�v(B)= ______________��
(2)��2mimĩA��ת����Ϊ______________
(3)�÷�Ӧ�Ļ�ѧ����ʽΪ____________________________
(4)6 min - 8 min��ֻ�ı��˷�Ӧ��ϵ��ijһ�����ʵ�Ũ�ȣ���ӦΪ_____(��ѡ����ĸ)
a.����A��Ũ�� b.��СB��Ũ�� c.����C��Ũ��
������ʵ�Ũ�ȸı���Ϊ______________ mol��L-1
(5) �������ͬ�����£�Ҫʹ�÷�Ӧ���淴Ӧ����ʼ���У��Ҵ�ƽ��ʱ���4min ʱ�����ʵ����������ȫ��ͬ�������B�����ʵ���Ũ�ȵ�ȡֵ��ΧΪ______________
(1)ǰ2mim�ڣ�v(B)= ______________��
(2)��2mimĩA��ת����Ϊ______________
(3)�÷�Ӧ�Ļ�ѧ����ʽΪ____________________________
(4)6 min - 8 min��ֻ�ı��˷�Ӧ��ϵ��ijһ�����ʵ�Ũ�ȣ���ӦΪ_____(��ѡ����ĸ)
a.����A��Ũ�� b.��СB��Ũ�� c.����C��Ũ��
������ʵ�Ũ�ȸı���Ϊ______________ mol��L-1
(5) �������ͬ�����£�Ҫʹ�÷�Ӧ���淴Ӧ����ʼ���У��Ҵ�ƽ��ʱ���4min ʱ�����ʵ����������ȫ��ͬ�������B�����ʵ���Ũ�ȵ�ȡֵ��ΧΪ______________
(1)0.30 mol��L-1��min-1
(2)12 %
(3)2A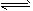 4B+C
4B+C
(4)C��1.00
(5)2.56 mol��L-1 < c��B���� 6.56 mol��L-1
(2)12 %
(3)2A
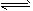 4B+C
4B+C (4)C��1.00
(5)2.56 mol��L-1 < c��B���� 6.56 mol��L-1

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 2NO2(g) ��H��+52.70 kJ��mol��1��
2NO2(g) ��H��+52.70 kJ��mol��1��
 2NO2(g) ��H��+52.70 kJ��mol��1��
2NO2(g) ��H��+52.70 kJ��mol��1��