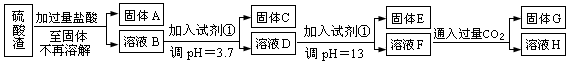
��Ŀ����
���Ṥҵ�з�����Ϊ����������ɷ�ΪSiO2��Fe2O3��Al2O3��MgO��ij̽����ѧϰС���ͬѧ������·����������������н���Ԫ�ص���ȡʵ�顣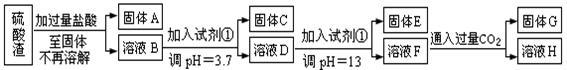
��֪��ҺpH=3.7ʱ��Fe3+�Ѿ�������ȫ��һˮ�ϰ����볣��Kb=1.8��10��5���䱥����Һ��c(OH��)ԼΪ1��10-3mol��L-1����ش�
��1��д��A������������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��2����������������ʹ���Լ��٣��Ʋ��Լ���Ӧ���� ����������ĸ��ţ�
| A���������� | B�������� | C����ˮ | D��ˮ |
��4��H�����ʵĻ�ѧʽ�� ��
��5��������ҺF��c(Mg2+)�� �� 25��ʱ��������þ��Ksp=5.6��10-12��
��1��SiO2+2NaOH=Na2SiO3+H2O��3�֣�
��2��A��2�֣�
��3��þ���ӳ�������ȫ�����������ܽⲻ��ȫ�ȣ�2�֣�
��4��NaHCO3��2�֣�
��5��5.6�� 10�C10 mol/L��3�֣�
����

(ÿ��3�ֹ���10�֣����Ṥҵ�з�����Ϊ����������ɷ�ΪSiO2��Fe2O3��Al2O3��MgO��ij̽����ѧϰС���ͬѧ������·����������������н���Ԫ�ص���ȡʵ�顣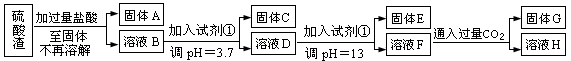
��֪��ҺpH=3.7ʱ��Fe3+�Ѿ�������ȫ��һˮ�ϰ����볣��Kb=1.8��10��5���䱥����Һ��c(OH��)ԼΪ1��10-3mol��L-1����ش�
��1��д��A������������Һ��Ӧ�Ļ�ѧ����ʽ������������������������������������
��2����������������ʹ���Լ��٣��Ʋ��Լ���Ӧ������������������������������������������ĸ��ţ�
| A���������� | B�������� | C����ˮ | D��ˮ |
��4��H�����ʵĻ�ѧʽ����������������������������
��5��������ҺF��c(Mg2+)��������������������25��ʱ��������þ��Ksp=5.6��10-12��
��10�֣����Ṥҵ�з�����Ϊ����������ɷ�ΪSiO2��Fe2O3��Al2O3��MgO��ij̽����ѧϰС���ͬѧ������·����������������н���Ԫ�ص���ȡʵ�顣 ��֪����Fe3+��Al3+��Mg2+������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��5.2��12.4��Al(OH)3��ȫ�ܽ��pHΪ11.8���ڳ����£����Ͱ�ˮ��pHԼΪ11��
��֪����Fe3+��Al3+��Mg2+������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��5.2��12.4��Al(OH)3��ȫ�ܽ��pHΪ11.8���ڳ����£����Ͱ�ˮ��pHԼΪ11��
��ش𣺣�1��д��A������������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��2�������������Լ���Ӧ���� ����������ĸ��ţ���
| A���������� | B�������� | C����ˮ | D��ˮ |
��
��4��������ҺF��c(Mg2+)�� �� 25��ʱ��������þ��Ksp=5.6��10-12����
��5����ҵ�����н�����C���պ�IJ�����KNO3��KOH������ȹ����Ʊ���ˮ��K2FeO4��ͬʱ���һ���������Σ���д���Ƶ�K2FeO4�Ļ�ѧ����ʽ�� ��
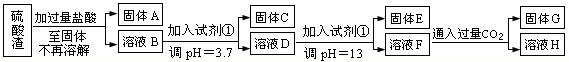
 ��֪����Fe3+��Al3+��Mg2+������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��5.2��12.4��Al(OH)3��ȫ�ܽ��pHΪ11.8���ڳ����£����Ͱ�ˮ��pHԼΪ11��
��֪����Fe3+��Al3+��Mg2+������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��5.2��12.4��Al(OH)3��ȫ�ܽ��pHΪ11.8���ڳ����£����Ͱ�ˮ��pHԼΪ11��