��Ŀ����
ij��ѧѧϰС��Ϊ�о�HA��HB��MOH������Ե����ǿ��,�������ʵ��:�����½�pH=2����������ҺHA��HB��pH=12��MOH����Һ��1 mL,�ֱ��ˮϡ�͵�1 000 mL,��pH�ı仯����Һ����Ĺ�ϵ��ͼ,��������������,��ش���������:
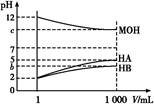
(1)HAΪ ��,HBΪ ��(�ǿ��������)��
(2)��c=9,��ϡ�ͺ��������Һ��,��ˮ�����������Ũ�ȵĴ�С˳��Ϊ (���ᡢ�ѧʽ��ʾ)��
(3)��c=9,��ϡ�ͺ��HA��Һ��MOH��Һȡ��������,��������Һ��c(A-)��c(M+)�Ĵ�С��ϵΪc(A-) (����ڡ�����С�ڡ����ڡ�)c(M+)��
(4)��b+c=14,��MOHΪ ��(�ǿ��������)����ϡ�ͺ��HB��Һ��MOH��Һȡ��������,���û����Һ��pH 7(����ڡ�����С�ڡ����ڡ�)��

(1)HAΪ ��,HBΪ ��(�ǿ��������)��
(2)��c=9,��ϡ�ͺ��������Һ��,��ˮ�����������Ũ�ȵĴ�С˳��Ϊ (���ᡢ�ѧʽ��ʾ)��
(3)��c=9,��ϡ�ͺ��HA��Һ��MOH��Һȡ��������,��������Һ��c(A-)��c(M+)�Ĵ�С��ϵΪc(A-) (����ڡ�����С�ڡ����ڡ�)c(M+)��
(4)��b+c=14,��MOHΪ ��(�ǿ��������)����ϡ�ͺ��HB��Һ��MOH��Һȡ��������,���û����Һ��pH 7(����ڡ�����С�ڡ����ڡ�)��
(1)ǿ �� (2)MOH=HA��HB
(3)���� (4)�� ����
(3)���� (4)�� ����
(1)pH��ͬ��������ϡ����ͬ�ı���,�����pH�仯С,��ͼ��֪pH=2������Һϡ��1 000��,HA��pH=5,��HA��ǿ��,��HB��Һϡ�ͺ�pH��5,��HB�����ᡣ
(2)��c=9,��MOH��ǿ��,����Һ����ˮ�������c(H+)=10-9 mol/L,��HA��ҺpH=5,c(H+)=10-5 mol/L,��c(OH-)=c(H+)ˮ����= mol/L=10-9 mol/L,��HB��ҺpH��5,��c(H+)��10-5 mol��L-1,��Һ��ˮ�������
mol/L=10-9 mol/L,��HB��ҺpH��5,��c(H+)��10-5 mol��L-1,��Һ��ˮ������� =c(OH-)��10-9 mol/L,����ˮ�����c(H+)��С˳��ΪMOH=HA��HB��
=c(OH-)��10-9 mol/L,����ˮ�����c(H+)��С˳��ΪMOH=HA��HB��
(3)��c=9,�൱��ǿ��HA��Һ��ǿ��MOH��Һ�������Ũ�Ȼ��,����ǡ����ȫ��Ӧ,��pH=7,��c(H+)=c(OH-),�ٸ��ݵ���غ��֪,c(H+)+c(M+)=c(OH-)+c(A-)�ɵó�c(A-)=c(M+)��
(4)��b+c=14,��b��5,��c��9,���ж�MOHΪ����,����ϡ��ǰHB��Һ��c(H+)=10-2 mol��L-1,ϡ�ͺ�HB��Һ��c(H+)=10-b mol��L-1,��ϡ��ǰMOH��Һ��c(OH-)=10-2 mol��L-1,ϡ�ͺ�MOH��Һ��c(OH-)=10-14+c mol��L-1=10-b mol��L-1,������ϡ��ǰ��,c(H+)��c(OH-)�ֱ��Ӧ���,�ݴ˿�֪:HB��MOH��ǿ���൱,������Һ����൱�ڵ������Ũ�ȵȵ���̶ȵ�HB��MOH���,����ǡ����ȫ��Ӧ����MB,��M+��B-��ˮ��̶���ͬ,�����û����ҺpH=7��
(2)��c=9,��MOH��ǿ��,����Һ����ˮ�������c(H+)=10-9 mol/L,��HA��ҺpH=5,c(H+)=10-5 mol/L,��c(OH-)=c(H+)ˮ����=
 mol/L=10-9 mol/L,��HB��ҺpH��5,��c(H+)��10-5 mol��L-1,��Һ��ˮ�������
mol/L=10-9 mol/L,��HB��ҺpH��5,��c(H+)��10-5 mol��L-1,��Һ��ˮ������� =c(OH-)��10-9 mol/L,����ˮ�����c(H+)��С˳��ΪMOH=HA��HB��
=c(OH-)��10-9 mol/L,����ˮ�����c(H+)��С˳��ΪMOH=HA��HB��(3)��c=9,�൱��ǿ��HA��Һ��ǿ��MOH��Һ�������Ũ�Ȼ��,����ǡ����ȫ��Ӧ,��pH=7,��c(H+)=c(OH-),�ٸ��ݵ���غ��֪,c(H+)+c(M+)=c(OH-)+c(A-)�ɵó�c(A-)=c(M+)��
(4)��b+c=14,��b��5,��c��9,���ж�MOHΪ����,����ϡ��ǰHB��Һ��c(H+)=10-2 mol��L-1,ϡ�ͺ�HB��Һ��c(H+)=10-b mol��L-1,��ϡ��ǰMOH��Һ��c(OH-)=10-2 mol��L-1,ϡ�ͺ�MOH��Һ��c(OH-)=10-14+c mol��L-1=10-b mol��L-1,������ϡ��ǰ��,c(H+)��c(OH-)�ֱ��Ӧ���,�ݴ˿�֪:HB��MOH��ǿ���൱,������Һ����൱�ڵ������Ũ�ȵȵ���̶ȵ�HB��MOH���,����ǡ����ȫ��Ӧ����MB,��M+��B-��ˮ��̶���ͬ,�����û����ҺpH=7��

��ϰ��ϵ�д�
�����Ŀ
 CH3COO-+H+
CH3COO-+H+ H++HS-
H++HS-
 CH3COO����H�������ڸ�ƽ������������ȷ���ǣ� ��
CH3COO����H�������ڸ�ƽ������������ȷ���ǣ� ��