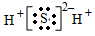��Ŀ����
������������Դ��Ҳ����Ҫ�Ļ���ԭ�ϣ����������������ⷽ��������������⣺
��1������һ��H2S�ȷֽⷨ����ӦʽΪ��H2S (g)  H2 (g)+S(g) ��H���ں����ܱ������У��ⶨH2S�ֽ��ת����(H2S����ʼŨ�Ⱦ�Ϊc mol/L)���ⶨ�����ͼ1����������a��ʾH2S��ƽ��ת�������¶ȹ�ϵ������b��ʾ��ͬ�¶��·�Ӧ������ͬʱ��δ�ﵽ��ѧƽ��ʱH2S��ת���ʡ�
H2 (g)+S(g) ��H���ں����ܱ������У��ⶨH2S�ֽ��ת����(H2S����ʼŨ�Ⱦ�Ϊc mol/L)���ⶨ�����ͼ1����������a��ʾH2S��ƽ��ת�������¶ȹ�ϵ������b��ʾ��ͬ�¶��·�Ӧ������ͬʱ��δ�ﵽ��ѧƽ��ʱH2S��ת���ʡ�
�١�H___________0���>����<����=������
����985��ʱ����Ӧ��t min�ﵽƽ�⣬��ʱH2S��ת����Ϊ40%����tmin�ڷ�Ӧ����v(H2)=______���ú�c��t�Ĵ���ʽ��ʾ����
����˵�����¶ȵ����ߣ�����b������a�ӽ���ԭ��_________________��
��2������������CaOΪ�����壬��������ʣ���C�ƣ���ˮ������Ӧ��ȡH2�������Ҫ��Ӧ���£�
I��C(s) + H2O (g) = CO (g) + H2(g) ��H = + 131.6 kJ/mol
II��CO (g) + H2O (g) = CO2 (g) + H2 (g) ��H = -43 kJ/mol
III��CaO(s) + CO2(g) = CaCO3(s) ��H = -178.3 kJ/mol
�ټ��㷴ӦC (s) +2H2O(g) +CaO(s) ==CaCO3 (s)+2H2 (g)�ġ�H=_____��������С�������1λ������K1��K2��K3�ֱ�Ϊ��ӦI��II��III��ƽ�ⳣ�����÷�Ӧ��ƽ�ⳣ��k=______����K1��K2��K3��ʾ����
�ڶ��ڿ��淴ӦC (s) +2H2O(g) +CaO(s)  CaCO3 (s)+2H2 (g)����ȡ���´�ʩ�������H2���ʵ���___�� ������ĸ��ţ�
CaCO3 (s)+2H2 (g)����ȡ���´�ʩ�������H2���ʵ���___�� ������ĸ��ţ�
A���ʵ��Ľ�����ϵ���¶� B��ʹ���������Ũ�Ⱦ��ӱ�
C������������������� D������CaO����
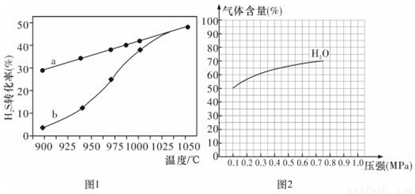
��ͼ2Ϊ��ӦI��һ���¶��£�ƽ��ʱ����������ٷֺ�����ѹǿ�仯�Ĺ�ϵͼ������Ӧ��ijһƽ��״̬ʱ�����c( H2O)=2c(H2)= 2c(CO)=2 mol��L���Ը���H2O������ٷֺ����仯���ߣ���������CO�ı仯����ʾ��ͼ��___________________
(3)��N2��H2Ϊ�缫��Ӧ���HCl-NH4C1Ϊ�������Һ��������ȼ�ϵ�أ��ŵ�����У���Һ��NH4+Ũ��������д���õ�ص�������Ӧʽ��___________________��
���ֶ�����Ԫ�ص�ijЩ����������ʾ���й�˵������ȷ����
Ԫ�� | Ԫ�ص������Ϣ |
M | ����������Ӧ��ˮ������������̬�⻯�ﷴӦ������ |
N | ԭ�ӵ�M���Ӳ�����3������ |
W | �ڶ�����Ԫ���У���ԭ�Ӱ뾶��� |
X | �������������ǵ��Ӳ�����2�����ҵͼ���������������̬�⻯�ﷴӦ����X�ĵ��ʺ�H2O |
Y | Ԫ���������������۵Ĵ�����Ϊ6 |
A. M����̬�⻯����л�ԭ�ԣ������£����⻯��ˮ��Һ��pH>7
B. W������������ȼ�պ�IJ������������Ӹ���֮��Ϊ1��2
C. ��N��Y���γɵĻ������д������Ӽ����������ӻ�����
D. N��W��X������������Ӧ��ˮ��������֮�����������Ӧ
ij�о�С�������ͼ��ʾʵ��װ�ã��гּ�����װ��ʡ�ԣ�����Cu(NO3)2 • 3H2O�����SOCl2�Ʊ�������ˮCu(NO3)2����֪SOC12�۵�-l05�桢�е�76�桢��ˮ����ˮ�����������������塣  .
.
��1��������c��������_________________��
����������ƿ�л����μ�SOC12ʱ�������_________(ѡ�a������b����a �� b������
��2��װ��A��Cu(NO3)2 • 3H2O��SOC12������Ӧ�Ļ�ѧ����ʽ��________________��
��3��װ��B��������________________��
��4��ʵ�����Ժ�����ͭмΪԭ���Ʊ�Cu(NO3)2 • 3H2O��ʵ�鷽�����£�
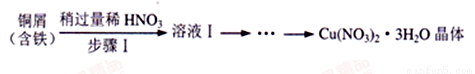
��֪�������������������������pH���±�
��ʼ������pH | ������ȫ��pH | |
Fe3+ | 1.1 | 3.2 |
Fe2+ | 5.8 | 8.8 |
Cu2+ • | 4.2 | 6.7 |
�ٲ���I������ϡHNO3�Թ�����Ŀ����_____________��
���벹����������ҺI�Ʊ�Cu(NO3)2 • 3H2O�����ʵ�鷽����
����ҺI�м���__________����ˮϴ�ӵõ�Cu(NO3)2 • 3H2O���塣


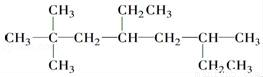 ������Ϊ________________________.
������Ϊ________________________. 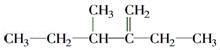 ������Ϊ______________________________.
������Ϊ______________________________.  �ṹ��ʽ��__________________________________.
�ṹ��ʽ��__________________________________.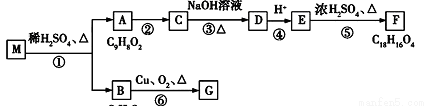
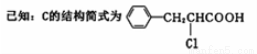
 B(g) ��H��������Ӧ�Ļ��ΪEakJ/mol���淴Ӧ�Ļ��ΪEbkJ/mol�����H=- (Ea-Eb) kJ/mol
B(g) ��H��������Ӧ�Ļ��ΪEakJ/mol���淴Ӧ�Ļ��ΪEbkJ/mol�����H=- (Ea-Eb) kJ/mol
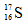 D. ������ӵĵ���ʽ��
D. ������ӵĵ���ʽ��