��Ŀ����
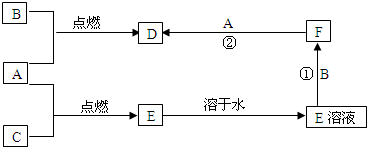
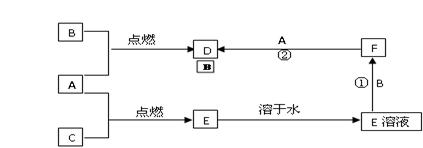
 A��B��CΪ���ֳ����ĵ��ʣ�����A��CΪ���壬BΪ����������A����ˮ���õ���Һ��ʹʯ����Һ�ȱ�����ɫ��F��ˮ��ҺΪdz��ɫ��Һ�����ǵĹ�ϵ��ͼ��
A��B��CΪ���ֳ����ĵ��ʣ�����A��CΪ���壬BΪ����������A����ˮ���õ���Һ��ʹʯ����Һ�ȱ�����ɫ��F��ˮ��ҺΪdz��ɫ��Һ�����ǵĹ�ϵ��ͼ��
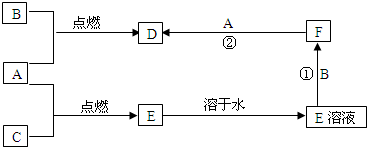
��1��д��A��B��D�Ļ�ѧʽ��
A��______��B��______��D��______
��2��д����Ӧ�٢ڵ����ӷ���ʽ
��______
��______
��3������D��ˮ��Һ�е������ӵķ����ǣ�______����D��Һ��μ���ķ�ˮ�л����һ�ֺ��ɫ�ij���Һ�壮��ͬѧ��Ϊ��Һ���еķ�ɢ������ֱ��Ӧ����1nm��100nm֮�䣬��֤��һ�뷨�ļ����ǣ�______
��4����ͼijͬѧ��A�ı�����Һװ�����Թܵ������ձ��У�����һ��ʱ������Թܵײ�����ɫ�������������Һ����ɫҲ�����ɫ����������Բ������������̽����
a����Ԥ�����������______��______
b��������Ԥ��д����֤ʵ�鷽����______��
�⣺��1��A����ˮ���õ���Һ��ʹʯ����Һ�ȱ�����ɫ����˵��AΪCl2����ˮ��Ӧ����HCl��HClO����ʹʯ����Һ�ȱ�����ɫ��F��ˮ��ҺΪdz��ɫ��Һ��˵��B�к���Fe2+���ܼ�����Cl2��Ӧ����D����DΪFeCl3������BΪFe����CΪH2��EΪHCl���ʴ�Ϊ��Cl2��Fe��FeCl3��
��2���ٷ�Ӧ��ΪFe������ķ�Ӧ����Ӧ�����ӷ���ʽΪFe+2H+�TFe2++H2�����ʴ�Ϊ��Fe+2H+�TFe2++H2����
��FeCl2���л�ԭ�ԣ�������������Ե�Cl2��Ӧ����FeCl3����Ӧ�����ӷ���ʽΪ2Fe2++Cl2�T2Fe3++2Cl-��
�ʴ�Ϊ��2Fe2++Cl2�T2Fe3++2Cl-��
��3������Fe3+������KSCN��Һ���۲���Һ�Ƿ��죬��������Ϊȡ������Һ���Թ��У��μ�����KSCN��Һ���۲���Һ�Ƿ���ɫ��������FeCl3��Һ�μӵ���ˮ�����������������壬���ж����ЧӦ��
�ʴ�Ϊ��ȡ������Һ���Թ��У��μ�����KSCN��Һ���۲���Һ�Ƿ���ɫ���Ƿ��ж��������
��4��a����ɫ�������Ϊ��������������Cl2��ˮ��Ӧ����HCl��HClO������HClO�IJ��ȶ��ԣ��ֽ������������ʴ�Ϊ��O2����H2��
b���������������������ʼ��Լ��飬������ȼ�գ�������ʹľ����ȼ��������������ˮ�¶�ס�Թܿڣ�ȡ�����ţ���ȼ�ŵ�ľ�������Թܿڣ��������ȼ��˵����H2�����ľ��ȼ�ո���˵����O2��
�ʴ�Ϊ����ˮ�¶�ס�Թܿڣ�ȡ�����ţ���ȼ�ŵ�ľ�������Թܿڣ��������ȼ��˵����H2�����ľ��ȼ�ո���˵����O2��
������A����ˮ���õ���Һ��ʹʯ����Һ�ȱ�����ɫ����˵��AΪCl2����ˮ��Ӧ����HCl��HClO����ʹʯ����Һ�ȱ�����ɫ��
F��ˮ��ҺΪdz��ɫ��Һ��˵��F�к���Fe2+���ܼ�����Cl2��Ӧ����D����DΪFeCl3������BΪFe����CΪH2��EΪHCl���Դ˽��1����2���⣻
��3������Fe3+������KSCN��Һ���۲���Һ�Ƿ��죻
��4��Cl2 ��ˮ��Ӧ����HCl��HClO��HClO���ȶ����ֽ�����������
���������⿼��������ƶϣ���Ŀ�ѶȲ�����ע�����㣺��A����ˮ���õ���Һ��ʹʯ����Һ�ȱ�����ɫ����˵��AΪCl2����F��ˮ��ҺΪdz��ɫ��Һ��˵��F�к���Fe2+��
��2���ٷ�Ӧ��ΪFe������ķ�Ӧ����Ӧ�����ӷ���ʽΪFe+2H+�TFe2++H2�����ʴ�Ϊ��Fe+2H+�TFe2++H2����
��FeCl2���л�ԭ�ԣ�������������Ե�Cl2��Ӧ����FeCl3����Ӧ�����ӷ���ʽΪ2Fe2++Cl2�T2Fe3++2Cl-��
�ʴ�Ϊ��2Fe2++Cl2�T2Fe3++2Cl-��
��3������Fe3+������KSCN��Һ���۲���Һ�Ƿ��죬��������Ϊȡ������Һ���Թ��У��μ�����KSCN��Һ���۲���Һ�Ƿ���ɫ��������FeCl3��Һ�μӵ���ˮ�����������������壬���ж����ЧӦ��
�ʴ�Ϊ��ȡ������Һ���Թ��У��μ�����KSCN��Һ���۲���Һ�Ƿ���ɫ���Ƿ��ж��������
��4��a����ɫ�������Ϊ��������������Cl2��ˮ��Ӧ����HCl��HClO������HClO�IJ��ȶ��ԣ��ֽ������������ʴ�Ϊ��O2����H2��
b���������������������ʼ��Լ��飬������ȼ�գ�������ʹľ����ȼ��������������ˮ�¶�ס�Թܿڣ�ȡ�����ţ���ȼ�ŵ�ľ�������Թܿڣ��������ȼ��˵����H2�����ľ��ȼ�ո���˵����O2��
�ʴ�Ϊ����ˮ�¶�ס�Թܿڣ�ȡ�����ţ���ȼ�ŵ�ľ�������Թܿڣ��������ȼ��˵����H2�����ľ��ȼ�ո���˵����O2��
������A����ˮ���õ���Һ��ʹʯ����Һ�ȱ�����ɫ����˵��AΪCl2����ˮ��Ӧ����HCl��HClO����ʹʯ����Һ�ȱ�����ɫ��
F��ˮ��ҺΪdz��ɫ��Һ��˵��F�к���Fe2+���ܼ�����Cl2��Ӧ����D����DΪFeCl3������BΪFe����CΪH2��EΪHCl���Դ˽��1����2���⣻
��3������Fe3+������KSCN��Һ���۲���Һ�Ƿ��죻
��4��Cl2 ��ˮ��Ӧ����HCl��HClO��HClO���ȶ����ֽ�����������
���������⿼��������ƶϣ���Ŀ�ѶȲ�����ע�����㣺��A����ˮ���õ���Һ��ʹʯ����Һ�ȱ�����ɫ����˵��AΪCl2����F��ˮ��ҺΪdz��ɫ��Һ��˵��F�к���Fe2+��

��ϰ��ϵ�д�
�����Ŀ
 A��B��CΪ���ֳ����ĵ��ʣ�����A��CΪ���壬BΪ����������A����ˮ���õ���Һ��ʹʯ����Һ�ȱ�����ɫ��F��ˮ��ҺΪdz��ɫ��Һ�����ǵĹ�ϵ��ͼ��
A��B��CΪ���ֳ����ĵ��ʣ�����A��CΪ���壬BΪ����������A����ˮ���õ���Һ��ʹʯ����Һ�ȱ�����ɫ��F��ˮ��ҺΪdz��ɫ��Һ�����ǵĹ�ϵ��ͼ��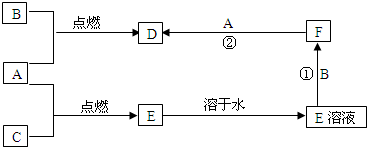
 ����ɫҲ�����ɫ����������Բ������������̽����
����ɫҲ�����ɫ����������Բ������������̽����