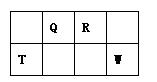
��Ŀ����
��15�֣�������Ԫ�����ڱ���һ���֣����ݸ�����Ԫ�ػش���������
��1��K��Ԫ�ط����� ��
��2��DԪ�ص���Χ�����Ų�ͼ�� �����仯�����У���������� ��
��3��CԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
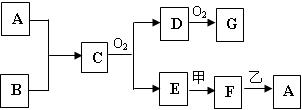
��4��A��L��B��L���ֱ����γ�ԭ�Ӹ�����Ϊ1��1�Ļ����A��L��1��1���Ļ������� ���ӣ���Ի�Ǽ��ԣ���B��L��1��1���Ļ�����ĵ���ʽ�� ��
��5��G��H��Lԭ�ӵĵ�һ�������ɸߵ��͵�˳���� ����Ԫ�ط��ţ���
��6��J���ʵ�һ�ְ�ɫͬ���칹��Ŀռ乹���� ��
��7��EԪ����Ԫ�����ڱ��е�λ���� �����仯�����У����У�2����3�۵�2�����ӣ���3�����ӱȽ��ȶ�����ԭ���� ��
��8��A��B�Ļ�������۵��A��L�Ļ�������۵�ߣ���ԭ���� ��
��9��A�ĵ�����L�ĵ�����B������������ˮ������Һ�����ȼ�յ�أ�д�����ֵ�صĸ����ĵ缫��Ӧʽ ��
��10��FԪ�ص������������MԪ�ص�����������ˮ���ﷴӦ�Ļ�ѧ����ʽ��
��

��1��K��Ԫ�ط����� ��
��2��DԪ�ص���Χ�����Ų�ͼ�� �����仯�����У���������� ��
��3��CԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��4��A��L��B��L���ֱ����γ�ԭ�Ӹ�����Ϊ1��1�Ļ����A��L��1��1���Ļ������� ���ӣ���Ի�Ǽ��ԣ���B��L��1��1���Ļ�����ĵ���ʽ�� ��
��5��G��H��Lԭ�ӵĵ�һ�������ɸߵ��͵�˳���� ����Ԫ�ط��ţ���
��6��J���ʵ�һ�ְ�ɫͬ���칹��Ŀռ乹���� ��
��7��EԪ����Ԫ�����ڱ��е�λ���� �����仯�����У����У�2����3�۵�2�����ӣ���3�����ӱȽ��ȶ�����ԭ���� ��
��8��A��B�Ļ�������۵��A��L�Ļ�������۵�ߣ���ԭ���� ��
��9��A�ĵ�����L�ĵ�����B������������ˮ������Һ�����ȼ�յ�أ�д�����ֵ�صĸ����ĵ缫��Ӧʽ ��
��10��FԪ�ص������������MԪ�ص�����������ˮ���ﷴӦ�Ļ�ѧ����ʽ��
��
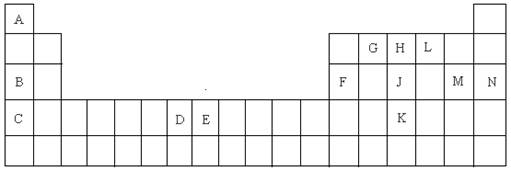
��1�� As ����2��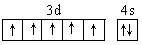 �� ��7 ����3��
�� ��7 ����3��  ��
��
��4�����ԣ� ����5�� N O C ����6�� �������壻
����5�� N O C ����6�� �������壻
��7�� �������ڵڢ��� �� Fe3����3d���Ϊ������ṹ�����ȶ� ��
��8�� NaH�����ӻ����H2O�ǹ��ۻ������9��H2��2e����2OH�D��2H2O ��
��10�� Al2O3��6HClO4��2Al(ClO4)3��3H2O ��9��10ÿ��2�֣�����ÿ��1�֣�
 �� ��7 ����3��
�� ��7 ����3��  ��
����4�����ԣ�
 ����5�� N O C ����6�� �������壻
����5�� N O C ����6�� �������壻��7�� �������ڵڢ��� �� Fe3����3d���Ϊ������ṹ�����ȶ� ��
��8�� NaH�����ӻ����H2O�ǹ��ۻ������9��H2��2e����2OH�D��2H2O ��
��10�� Al2O3��6HClO4��2Al(ClO4)3��3H2O ��9��10ÿ��2�֣�����ÿ��1�֣�
��

��ϰ��ϵ�д�
�����Ŀ
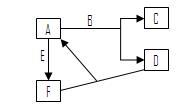
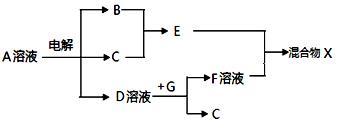
 ��
��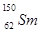 ��ϡ��Ԫ�أ������й�˵������ȷ����
��ϡ��Ԫ�أ������й�˵������ȷ����