��Ŀ����
����Ŀ��һ���¶��£���2 L���ܱ������У�X��Y��Z�������������ʱ��仯����������ͼ��ʾ��

��1���ӷ�Ӧ��ʼ��10 sʱ����Z��ʾ�ķ�Ӧ����Ϊ________��X�����ʵ���Ũ�ȼ�����______��Y��ת����Ϊ________��
��2���÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
��3��10 s���ijһʱ��(t1)�ı��������������������ʱ��ı仯ͼ������ͼ��ʾ��������˵�����ϸ�ͼ�����________��
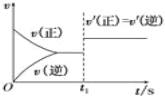
A��t1ʱ�̣�������X��Ũ��
B��t1ʱ�̣���������ϵ�¶�
C��t1ʱ�̣���С���������
D��t1ʱ�̣�ʹ���˴���
���𰸡�
��1�� 0.079 mol/(L��s)��0.395 mol/L��79.0%
��2�� X(g)��Y(g) ![]() 2Z(g)��
2Z(g)��
��3�� CD
��������
���������ͼ�������֪��ʼ��Ϊ��X=1.2mol��Y=1.00mol��Z=1.58mol��10s�ﵽƽ��״̬xyz�����ʵ���Ϊ��0.41mol��0.21mol��1.58mol����X=1.2mol-0.41mol=0.79mol����Y=1.00mol-0.21mol=0.79mol����Z=1.58mol��
��1����Ӧ����v(Z)=![]() =
=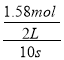 =0.079mol/(Ls)��X��Ũ�ȼ�Сc=
=0.079mol/(Ls)��X��Ũ�ȼ�Сc=![]() =
=![]() =0.395mol/L��Yת����=
=0.395mol/L��Yת����=![]() ��100%=
��100%=![]() ��100%=79%���ʴ�Ϊ��0.079mol/(Ls)��0.395mol/L��79%��
��100%=79%���ʴ�Ϊ��0.079mol/(Ls)��0.395mol/L��79%��
��2��n(X)��n(Y)��n(Z)=0.79��0.79��1.58=1��1��2����Ӧ�Ļ�ѧ����ʽ������֮�ȵ������ʲμӷ�Ӧ�����ʵ���֮�ȣ���֪��ѧ����ʽΪ��X+Y![]() 2Z���ʴ�Ϊ��X+Y
2Z���ʴ�Ϊ��X+Y![]() 2Z
2Z
��3����ͼ���֪���淴Ӧ��������ƽ��û�ƶ���A��t1ʱ�̣�������X��Ũ�ȣ�ƽ�����ƣ����������⣬��A����B��t1ʱ�̣���������ϵ�¶ȣ�ƽ���ƶ������������⣬��B����C��t1ʱ�̣���С����������������������ڷ�Ӧǰ���������ȣ�ƽ�ⲻ�ƶ����������⣬��C��ȷ��D��t1ʱ�̣�ʹ���˴�������������ƽ�ⲻ�ƶ����������⣬��D��ȷ���ʴ�Ϊ��CD��

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�����Ŀ������һƿ���ʼ��ҵĻ�����֪���ҵ�ijЩ���������ʾ��
���� | ����ʽ | �۵�(��) | �е�(��) | �ܶ� (g��cm��3) | ˮ�е��ܽ��� |
�� | C3H6O2 | ��98 | 57.5 | 0.93 | ���� |
�� | C4H8O2 | ��84 | 77 | 0.90 | ���� |
�ݴˣ������ҷ������ѷ�����
A����ȡ�� B�������� C������ D����Һ��

