��Ŀ����
��������(NaClO2)��һ����Ҫ�ĺ�������������Ҫ����ˮ�������Լ�ɰ�ǵ�Ư��ɱ������һ�����������������£�

�ش��������⣺
(1) д������Ӧ������������ClO2�Ļ�ѧ����ʽ��________________________��
(2) ����⡱����ʳ��ˮ�ɴ���ˮ���ƶ��ɣ�����ʱΪ��ȥMg2����Ca2����Ҫ������Լ��ֱ�Ϊ___________________��___________________��

(3) ��������(NaClO2)��ˮ��Һ�п�����ClO2��HClO2��ClO ��Cl���ȣ�����HClO2��ClO2������Ư�����á����ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ(Cl��û�л���)����ʹ�ø�Ư��������pH________(����ڡ��� ���ڡ���С�ڡ�)3��
��Cl���ȣ�����HClO2��ClO2������Ư�����á����ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ(Cl��û�л���)����ʹ�ø�Ư��������pH________(����ڡ��� ���ڡ���С�ڡ�)3��
(4) Ϊ�˲ⶨNaClO2��3H2O�Ĵ��ȣ�ȡ�����ϳɲ�Ʒ10.00 g����ˮ���1000 mL��Һ��ȡ��20.00 mL��Һ����ƿ�У��ټ������������ữ��KI��Һ����ַ�Ӧ�����2��3�ε�����Һ����0.2640 mol��L��1Na2S2O3����Һ�ζ�������ȥNa2S2O3����Һ20.00 mL��ͨ������ȷ���ò�Ʒ�����Ƿ�ϸ�(�ϸ���90%����)(д���������)__________
��ʾ�� 2Na2S2O3��I2===Na2S4O6��2NaI��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

 4NO(g)+CO2(g)+2H2O(g) ��H=-574kJ/mol
4NO(g)+CO2(g)+2H2O(g) ��H=-574kJ/mol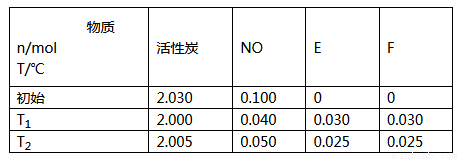
 2CO2+N2��ij�¶�ʱ����1L�ܱ������г���0.1molCO��0.1mol NO��5sʱ��Ӧ�ﵽƽ�⣬���NO��Ũ��Ϊ0.02mol/L����Ӧ��ʼ��ƽ��ʱ��NO��ƽ����Ӧ����v(NO)=________�������¶��£�ijʱ�̲��CO��NO��N2��CO2��Ũ�ȷֱ�Ϊ0.01mol/L��amol/L��0.01mol/L��0.04mol/L��Ҫʹ��Ӧ������Ӧ������У�a��ȡֵ��ΧΪ_____________��
2CO2+N2��ij�¶�ʱ����1L�ܱ������г���0.1molCO��0.1mol NO��5sʱ��Ӧ�ﵽƽ�⣬���NO��Ũ��Ϊ0.02mol/L����Ӧ��ʼ��ƽ��ʱ��NO��ƽ����Ӧ����v(NO)=________�������¶��£�ijʱ�̲��CO��NO��N2��CO2��Ũ�ȷֱ�Ϊ0.01mol/L��amol/L��0.01mol/L��0.04mol/L��Ҫʹ��Ӧ������Ӧ������У�a��ȡֵ��ΧΪ_____________��
 2SO3(g) ��H=��158.4kJ��mol��1
2SO3(g) ��H=��158.4kJ��mol��1 Si3N4��12HCl���йظ÷�Ӧ˵����ȷ����
Si3N4��12HCl���йظ÷�Ӧ˵����ȷ����