��Ŀ����
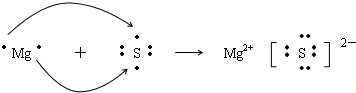
�ش�����������1���Լ�����FeSO4��Һ�м���NaOH��Һʱ�ķ�Ӧ������ɰ�˳��Ӧ�Ļ�ѧ����ʽ�������ӷ���ʽ��
����
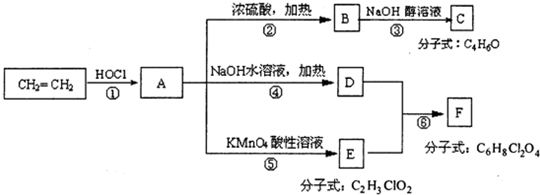
��2����֪��ij��Ӧ��ϵ�е������У�Fe��OH��3��NaClO��NaOH��NaCl��Na2FeO4���������ƣ���H2O ����Na2FeO4��H2O�Dz����еĶ��֣�
��д������ƽ��Ӧ�Ļ�ѧ����ʽ��
���ڸ÷�Ӧ�У��õ��ӵ�������
���ڸ÷�Ӧ�У�ÿ��3mol�ĵ���ת�ƣ����ģ����ɣ�NaOH�����ʵ�����
���ɷ���ʽ�ж���֪��NaClO��Na2FeO4��������ǿ��˳����
��������1���������������ȶ��ܱ������е���������Ϊ����������
��2�����ݻ��ϼ۵�����������Na2FeO4�е���Ԫ����Դ�ڷ�Ӧ��Fe��OH��3��Fe��OH��3����ԭ�������Ӧ��������ΪNaClO�����û��ϼ����������ƽ��ת�Ƶ��������뻹ԭ��ʧȥ�ĵ�����Ҳ�����������õ��ĵ��������������������Դ�����������������ԣ�
��2�����ݻ��ϼ۵�����������Na2FeO4�е���Ԫ����Դ�ڷ�Ӧ��Fe��OH��3��Fe��OH��3����ԭ�������Ӧ��������ΪNaClO�����û��ϼ����������ƽ��ת�Ƶ��������뻹ԭ��ʧȥ�ĵ�����Ҳ�����������õ��ĵ��������������������Դ�����������������ԣ�
�����1����FeSO4��Һ�м���NaOH��Һʱ��Fe2+��OH-��Ӧ����Fe��OH��2��ɫ������Fe��OH��2���ȶ����ᱻ�����е���������ΪFe��OH��3���ɫ�������漰����ʽΪFe2++2OH-=Fe��OH��2����4Fe��OH��2+O2+2H2O=4Fe��OH��3��
�ʴ�Ϊ���ȳ��ְ�ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ������Fe2++2OH-=Fe��OH��2����4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��2���ٸ���������ԭ��Ӧԭ����֪������Na2FeO4�е���Ԫ����Դ��Fe��OH��3�����Fe��OH��3��ԭ������ԭ��ʧ���ӣ����Ӧ��������ΪNaClO���������õ��ӣ���ԭ����ΪNaCl������ԭ���غ����ȷ��������������Ӧ����ݻ��ϼ����������ƽ�ã�3NaClO+2Fe��OH��3+4NaOH=3NaCl+2Na2FeO4+5H2O��
�ʴ�Ϊ��3NaClO+2Fe��OH��3+4NaOH=3NaCl+2Na2FeO4+5H2O��
���ɢٷ�����֪NaClOΪ��������Fe��OH��3Ϊ��ԭ�����ʴ�Ϊ��NaClO��Fe��OH��3��
��ת�Ƶ��������ڻ�ԭ��ʧȥ�ĵ�����������3NaClO+2Fe��OH��3+4NaOH=3NaCl+2Na2FeO4+5H2O�ͻ��ϼ۱仯��֪��
Fe��OH��3��Na2FeO4��3e-��2NaOH
1 1 3 2
1mol 1mol 3mol 2mol
�ʴ�Ϊ��2mol��
��NaClOΪ��������Na2FeO4��������������������Դ�����������������ԣ����������ԣ�NaClO��Na2FeO4��
�ʴ�Ϊ��NaClO��Na2FeO4��
�ʴ�Ϊ���ȳ��ְ�ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ������Fe2++2OH-=Fe��OH��2����4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��2���ٸ���������ԭ��Ӧԭ����֪������Na2FeO4�е���Ԫ����Դ��Fe��OH��3�����Fe��OH��3��ԭ������ԭ��ʧ���ӣ����Ӧ��������ΪNaClO���������õ��ӣ���ԭ����ΪNaCl������ԭ���غ����ȷ��������������Ӧ����ݻ��ϼ����������ƽ�ã�3NaClO+2Fe��OH��3+4NaOH=3NaCl+2Na2FeO4+5H2O��
�ʴ�Ϊ��3NaClO+2Fe��OH��3+4NaOH=3NaCl+2Na2FeO4+5H2O��
���ɢٷ�����֪NaClOΪ��������Fe��OH��3Ϊ��ԭ�����ʴ�Ϊ��NaClO��Fe��OH��3��
��ת�Ƶ��������ڻ�ԭ��ʧȥ�ĵ�����������3NaClO+2Fe��OH��3+4NaOH=3NaCl+2Na2FeO4+5H2O�ͻ��ϼ۱仯��֪��
Fe��OH��3��Na2FeO4��3e-��2NaOH
1 1 3 2
1mol 1mol 3mol 2mol
�ʴ�Ϊ��2mol��
��NaClOΪ��������Na2FeO4��������������������Դ�����������������ԣ����������ԣ�NaClO��Na2FeO4��
�ʴ�Ϊ��NaClO��Na2FeO4��
���������⿼�������仯�����֪ʶ�Ƚϻ������ѶȲ����漰������ԭ��Ӧ����ʽ����д��ת�Ƶ������ļ������ѵ㣬������ԭ��Ӧ�����ֵ���ǻ��ϼۣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ