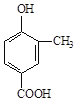
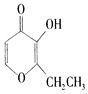
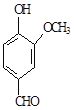
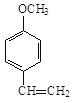
��Ŀ����
����Ŀ����A����ϩ B���Ҵ� C������ D����֬ E�������� F����ά�������л�����ѡ����ʵ����ʣ����������ڿո��ڣ�
��1�������������____________��
��2������̼������Һ������Ӧ�ų�������̼�������____________��
��3����ˮ�����ɸ�֬������͵���____________��
��4�������л��߷��ӻ��������____________��
��5��75%(�������)��____________��Һ������ҽ��������
��6�������˵���Һ�ܷ���������Ӧ������Һ�ﺬ��____________��
���𰸡� A C D F B E
��������������������⿼���л���ķ��࣬��ϩ���Ҵ������ᡢ��֬�������ǡ���ά�ص����ʺ���;����ϩ�ķ���ʽΪC2H4���ṹ��ʽΪCH2=CH2���Ҵ��ķ���ʽΪC2H6O���ṹ��ʽΪCH3CH2OH������ķ���ʽΪC2H4O2���ṹ��ʽΪCH3COOH����֬�Ǹ�֬����ĸ����������Ԫ��ΪC��H��O�������ǵķ���ʽΪC6H12O6���ṹ��ʽΪCH2OH��CHOH��4CHO����ά�صķ���ʽΪ��C6H10O5��n��
��1������̼��������Ԫ�ص��л����Ϊ�����������������ϩ����ѡA��
��2������̼������Һ��Ӧ�ų�������̼��������������ԣ�Ϊ���ᣬ��Ӧ�Ļ�ѧ����ʽΪ��2CH3COOH+Na2CO3��2CH3COONa+H2O+CO2������ѡC��
��3����֬������������ˮ�����ɸ�֬������ͣ���ѡD��
��4����ά��������Ȼ�л��߷��ӻ������ѡF��
��5��75%���Ҵ���Һ������ҽ����������ѡB��
��6�������˵���Һ�ܷ���������Ӧ������Һ�е����ʺ�ȩ��������Һ�к������ǣ���Ӧ�Ļ�ѧ����ʽΪ��CH2OH��CHOH��4CHO+2Ag��NH3��2OH![]() CH2OH��CHOH��4COONH4+2Ag��+3NH3+H2O����ѡE��
CH2OH��CHOH��4COONH4+2Ag��+3NH3+H2O����ѡE��

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д�