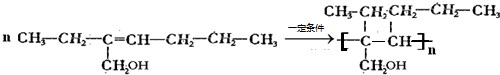��Ŀ����
��.1����������л�����з��࣬����ȷ�𰸣���ţ���д�ڱ����Ӧ�����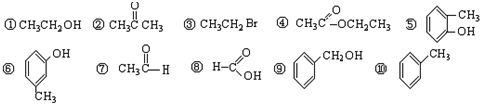
2������10�������У���Ϊλ���칹����ǣ���д��ţ�
����ϵͳ������д�������������ƣ�
1����CH3��2CHCH=CHCH��C2H5��CH2CH3
2��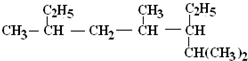
������A��ϵͳ����Ϊ��1-��ϩ������ṹ��ʽΪ

| ������ | ±���� | �� | �� | ȩ | ͪ | ���� | �� |
�ݢ�
�ݢ�
������ϵͳ������д�������������ƣ�
1����CH3��2CHCH=CHCH��C2H5��CH2CH3
2��
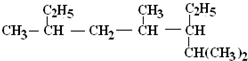
������A��ϵͳ����Ϊ��1-��ϩ������ṹ��ʽΪ
CH2=CH-CH2-CH3
CH2=CH-CH2-CH3
����д����û����ﺬ����ͬ�����ŵ���������ͬ���칹��Ľṹ��ʽ����Ӧ���ƣ���������1�������л����к��еĹ�����ȷ���л�������ࣻ
2�������ͬ�������е�ȡ����������ţ�����̼̼˫������������̼�ܣ�̼����̼�����ϵ�λ�ò�ͬ����Щ�������λ���칹�壻
��1������ϩ����ϵͳ��������
2������������ϵͳ��������
����������ṹ��ʽ�Ķ�Ӧ��ϵ����д�ṹ������д������λ���칹�����д��������д��������
2�������ͬ�������е�ȡ����������ţ�����̼̼˫������������̼�ܣ�̼����̼�����ϵ�λ�ò�ͬ����Щ�������λ���칹�壻
��1������ϩ����ϵͳ��������
2������������ϵͳ��������
����������ṹ��ʽ�Ķ�Ӧ��ϵ����д�ṹ������д������λ���칹�����д��������д��������
����⣺��1������к��б������ʢ��Ƿ�����������к���±��ԭ�ӣ��ʢ���±��������١����к��д��ǻ����ʢ١������ڴ�����ݡ����к��з��ǻ����ʢݡ������ڷӣ�����к���ȩ�����ʢ�����ȩ������к����ʻ����ʢ�����ͪ������к����Ȼ����ʢ����������к����������ʢ��������ʴ�Ϊ��
2��������ͬ���ڷӣ���ȡ����������ŵ�λ�ò�ͬ����������Ϊλ���칹�壬�ʴ�Ϊ������ޣ�
��1����CH3��2CHCH=CHCH��C2H5��CH2CH3��ϵͳ������Ϊ��2-��-5-�һ�-3-��ϩ���ʴ�Ϊ��2-��-5-�һ�-3-��ϩ��
2��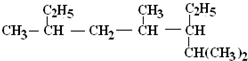 ��ϵͳ������Ϊ��2��4��6-����-3-�һ����飬�ʴ�Ϊ��2��4��6-����-3-�һ����飻
��ϵͳ������Ϊ��2��4��6-����-3-�һ����飬�ʴ�Ϊ��2��4��6-����-3-�һ����飻
��1-��ϩ���Ľṹ��ʽΪ��CH2=CHCH2CH3���û����ﺬ����ͬ�����ŵ���������ͬ���칹��Ľṹ��ʽ����Ӧ����Ϊ��CH3CH=CHCH3��2-��ϩ��CH2=C��CH3��2��2-��-1-��ϩ��
�ʴ�Ϊ��CH2=CHCH2CH3��CH3CH=CHCH3��2-��ϩ��CH2=C��CH3��2��2-��-1-��ϩ��
| ������ | ±���� | �� | �� | ȩ | ͪ | ���� | �� |
| �� | �� | �٢� | �ݢ� | �� | �� | �� | �� |
��1����CH3��2CHCH=CHCH��C2H5��CH2CH3��ϵͳ������Ϊ��2-��-5-�һ�-3-��ϩ���ʴ�Ϊ��2-��-5-�һ�-3-��ϩ��
2��
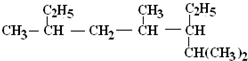 ��ϵͳ������Ϊ��2��4��6-����-3-�һ����飬�ʴ�Ϊ��2��4��6-����-3-�һ����飻
��ϵͳ������Ϊ��2��4��6-����-3-�һ����飬�ʴ�Ϊ��2��4��6-����-3-�һ����飻��1-��ϩ���Ľṹ��ʽΪ��CH2=CHCH2CH3���û����ﺬ����ͬ�����ŵ���������ͬ���칹��Ľṹ��ʽ����Ӧ����Ϊ��CH3CH=CHCH3��2-��ϩ��CH2=C��CH3��2��2-��-1-��ϩ��
�ʴ�Ϊ��CH2=CHCH2CH3��CH3CH=CHCH3��2-��ϩ��CH2=C��CH3��2��2-��-1-��ϩ��
������������Ҫ�������л�����з��ࡢ������ͬ���칹��ĸ�������ʵķ���ʱ���ݹ����ŵIJ�ͬ�����ʷ�������ݣ�

��ϰ��ϵ�д�
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�����Ŀ
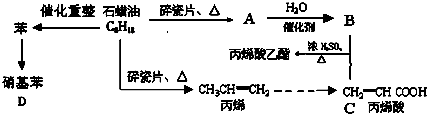
 ��������һ����Ҫ���л�����ԭ�ϣ��Ʊ��������ԭ����95%�Ҵ���80%���ᣨ������ˮϡ��Ũ���ᣩ����ϸ���廯�Ʒ�ĩ�ͼ������Ƭ���÷�Ӧ��ԭ�����£�
��������һ����Ҫ���л�����ԭ�ϣ��Ʊ��������ԭ����95%�Ҵ���80%���ᣨ������ˮϡ��Ũ���ᣩ����ϸ���廯�Ʒ�ĩ�ͼ������Ƭ���÷�Ӧ��ԭ�����£�NaBr+H2SO4��NaHSO4+HBr
CH3CH2OH+HBr
| ���� |
ij����С������ʵ�����Ʊ��������װ����ͼ�����������
| ���� ���� |
�Ҵ� | ������ | 1��2-�������� | ���� | Ũ���� |
| �ܶ�/g?cm-3 | 0.79 | 1.46 | 2.2 | 0.71 | 1.84 |
| �۵㣨�棩 | -130 | -119 | 9 | -116 | 10 |
| �е㣨�棩 | 78.5 | 38.4 | 132 | 34.6 | 338 |
| ��ˮ�е��ܽ�ȣ�g/100gˮ�� | ���� | 0.914 | 1 | 7.5 | ���� |
��1������ҩƷ֮ǰ�����IJ����ǣ�
��2��װ��B�������dz���ʹ���������������һ��Ŀ����
��3����Ӧʱ�п�������SO2��һ�ֺ���ɫ���壬��ѡ������������Һ��ȥ�����壬�йص����ӷ���ʽ��
��4��ʵ���в���80%���ᣬ��������98%Ũ���ᣬһ������Ϊ�˼��ٸ���Ӧ����һ������Ϊ��
��5���ֲ�Ʒ�к��е���Ҫ�л�Һ��������
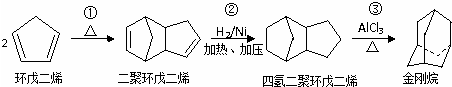
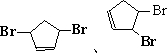

 ����д��A���п��ܵĽṹ��ʽ
����д��A���п��ܵĽṹ��ʽ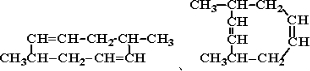

 ��X����±ԭ�ӣ�
��X����±ԭ�ӣ� +
+
 +H2O
+H2O