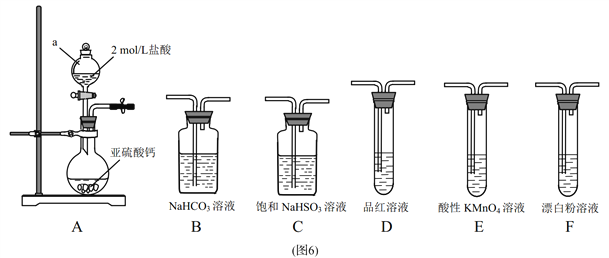
��Ŀ����
����Ŀ��NaClO2����Ҫ�ĺ��Ȼ�����Ʒ����ҵ������NaClO2�ж��ַ�����ij��ҵ����������ͼ���£�

��1��NaClO2��Ԫ�صĻ��ϼ�Ϊ___________��
��2�������������ϵĵ缫��ӦʽΪ_______________��
��3����Ӧ�����з�����Ӧ�Ļ�ѧ����ʽΪ___________����Ӧ�����з����ķ�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ_________________��
��4�������Т١����ڹ�ҵ�����е���ʵ������__________����֪NaClO2������Һ���¶ȵ���38��ʱ����NaClO2��3H2O���壬����38��ʱ����NaClO2���壬����60��ʱNaClO2�ֽ⣬��������л�õ�NaClO2��Һ�еõ�NaClO2����IJ���Ϊ��_______��30��-60����ˮϴ�ӡ�����60����
��5����Ʒ��NaClO2�����IJⶨ��ȷ��ȡa g NaClO2��Ʒ������ƿ�У�������ˮ�ܽ���������һ��Ũ�ȵ�KI��Һ��������Һ��Ȼ���ٵμ�ϡ����ʹ��Ʒ�е�NaClO2ǡ��ȫ������ԭΪNaCl������c mol/L Na2S2O3��Һ�ζ���������ӦI2+2S2O32-=2I-+S4O62-������Na2S2O3��Һ�����ΪV mL��
�� ʢװNa2S2O3��ҺӦѡ��______ʽ�ζ��ܣ�����ζ��յ�ʱ������Ϊ________.
�� ��Ʒ��NaClO2����������w(NaClO2)=______���ú�c��V��a�Ĵ���ʽ��ʾ����
���𰸡� +3 Cl-+3H2O-6e-=ClO3-+6H+ 2NaClO3+4HCl=2NaCl+2ClO2��+Cl2��+2H2O 2:1 ���ԭ�������ʣ����������ɱ� ��ѹ�����£���55��ʱ������Ũ�������ȹ��� �� ��ɫ��ʧ����30s�ڲ���ԭ 2.2625cV/a%
����������1��NaClO2��Na��O�Ļ��ϼ۷ֱ���+1��-2�ۣ�����ݻ��ϼ۴�����Ϊ0��֪��Ԫ�صĻ��ϼ�Ϊ+3�ۡ���2�������������Ϸ���ʧȥ���ӵ�������Ӧ�����������������ƣ��������ĵ缫��ӦʽΪCl-+3H2O-6e-��ClO3-+6H+����3����Ӧ�����������ƺ��Ȼ��ⷴӦ�����������������Ⱥ��Ȼ��ƣ���Ӧ�Ļ�ѧ����ʽΪ2NaClO3+4HCl��2NaCl+2ClO2��+Cl2��+2H2O����Ӧ�����з����ķ�Ӧ���������Ƕ������ȣ���Ԫ�ػ��ϼ۴�+4�۽��͵�+3�ۣ�˫��ˮ�ǻ�ԭ����������������������˸��ݵ��ӵ�ʧ�غ��֪�������뻹ԭ�����ʵ���֮��Ϊ2:1����4���������̢١����е�����ת����֪�ڹ�ҵ�����е���ʵ���������ԭ�������ʣ����������ɱ���������֪��Ϣ��֪�������л�õ�NaClO2��Һ�еõ�NaClO2����IJ���Ϊ����ѹ�����£���55��ʱ������Ũ�������ȹ��ˣ���30�桫60����ˮϴ�ӡ�����60������5����Na2S2O3��Һ�Լ��ԣ�ʢװNa2S2O3��ҺӦѡ�ü�ʽ�ζ��ܡ�������������ɫ����ζ��յ�ʱ������Ϊ�������һ�α�Һ����ɫ��ʧ����30s�ڲ���ԭ����������������Ƶ����ʵ�����0.001cVmol��ת��0.001cVmol���ӣ����ݵ��ӵ�ʧ�غ��֪��Ʒ��NaClO2�����ʵ�����0.001cVmol��4����������0.001cVmol/4��90.5g/mol��0.022625g������������w(NaClO2)��0.022625g /ag��100%��2.2625cV/a%��