МвДїДЪИЭ
ЈЁ18·ЦЈ©ПВ±нОЄФЄЛШЦЬЖЪ±нЦРµДТ»Ії·ЦЈ¬±нЦРБРіцБЛ11ЦЦФЄЛШФЪЦЬЖЪ±нЦРµДО»ЦГЈ¬°ґТЄЗуНкіЙПВБРёчРЎМвЎЈ

(1) »ЇС§РФЦКЧоІ»»оЖГµДФЄЛШКЗ ЈЁМоФЄЛШ·ыєЕ»т»ЇС§КЅЈ¬ПВН¬Ј©Ј¬
·ЗЅрКфРФЧоЗїµДФЄЛШКЗ ЎЈ
ЅрКфРФЧоЗїµДµҐЦКУлЛ®·ґУ¦µДАлЧУ·ЅіМКЅКЗ
ЎЈ
(2) ўЩўЫўЭИэЦЦФЄЛШµДЧоёЯјЫСх»ЇОп¶ФУ¦µДЛ®»ЇОпЦРЈ¬јоРФЧоЗїµД»ЇєПОпµД»ЇС§КЅКЗ
(3) ўЩўЪўЫИэЦЦФЄЛШµДФЧУ°лѕ¶УЙґуµЅРЎµДЛіРтКЗ Јѕ Јѕ ЎЈ
(4) ДіФЄЛШµДЧоёЯјЫСх»ЇОп¶ФУ¦µДЛ®»ЇОпјИДЬУлЛб·ґУ¦ЙъіЙСОєНЛ®Ј¬УЦДЬєНјо·ґУ¦
ЙъіЙСОєНЛ®Ј¬ёГФЄЛШµДЧоёЯјЫСх»ЇОпєНСОЛб·ґУ¦µДАлЧУ·ЅіМКЅОЄ
ЎЈ
ПтёГФЄЛШєНўаєЕФЄЛШРОіЙµД»ЇєПОпµДИЬТєЦРЈ¬»єВэµОјУ°±Л®ЦБ№эБїЈ¬ІъЙъµДКµСйПЦПуКЗ Ј¬УР№Ш·ґУ¦µД»ЇС§·ЅіМКЅОЄ ЎЈЈЁ5Ј©ўЮФЄЛШРОіЙµДµҐЦКУлЕЁПхЛб·ўЙъ»ЇС§·ґУ¦µД»ЇС§·ЅіМКЅОЄ
ЎЈ

(1) »ЇС§РФЦКЧоІ»»оЖГµДФЄЛШКЗ ЈЁМоФЄЛШ·ыєЕ»т»ЇС§КЅЈ¬ПВН¬Ј©Ј¬
·ЗЅрКфРФЧоЗїµДФЄЛШКЗ ЎЈ
ЅрКфРФЧоЗїµДµҐЦКУлЛ®·ґУ¦µДАлЧУ·ЅіМКЅКЗ
ЎЈ
(2) ўЩўЫўЭИэЦЦФЄЛШµДЧоёЯјЫСх»ЇОп¶ФУ¦µДЛ®»ЇОпЦРЈ¬јоРФЧоЗїµД»ЇєПОпµД»ЇС§КЅКЗ
(3) ўЩўЪўЫИэЦЦФЄЛШµДФЧУ°лѕ¶УЙґуµЅРЎµДЛіРтКЗ Јѕ Јѕ ЎЈ
(4) ДіФЄЛШµДЧоёЯјЫСх»ЇОп¶ФУ¦µДЛ®»ЇОпјИДЬУлЛб·ґУ¦ЙъіЙСОєНЛ®Ј¬УЦДЬєНјо·ґУ¦
ЙъіЙСОєНЛ®Ј¬ёГФЄЛШµДЧоёЯјЫСх»ЇОпєНСОЛб·ґУ¦µДАлЧУ·ЅіМКЅОЄ
ЎЈ
ПтёГФЄЛШєНўаєЕФЄЛШРОіЙµД»ЇєПОпµДИЬТєЦРЈ¬»єВэµОјУ°±Л®ЦБ№эБїЈ¬ІъЙъµДКµСйПЦПуКЗ Ј¬УР№Ш·ґУ¦µД»ЇС§·ЅіМКЅОЄ ЎЈЈЁ5Ј©ўЮФЄЛШРОіЙµДµҐЦКУлЕЁПхЛб·ўЙъ»ЇС§·ґУ¦µД»ЇС§·ЅіМКЅОЄ
ЎЈ
(16·Ц) ЈЁ1Ј© ArЈ¬FЈ¬2K+2H2O=2K++2OH-+H2Ўь
ЈЁ2Ј© NaOHЈ¬ ЈЁ3Ј© KЈѕNaЈѕMgЈ¬
ЈЁ4Ј© Al2O3+6H+="2" Al3++3H2O
ПИіцПЦ°ЧЙ«іБµнЈ¬ІўЦрЅҐФц¶а AlCl3+3NH3Ў¤ H2O=Al(OH)3Ўэ+ 3NH4ClЈ¬
ЈЁ5Ј©CЈ«4HNO3(ЕЁ)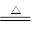 CO2ЎьЈ«4NO2ЎьЈ«2H2O
CO2ЎьЈ«4NO2ЎьЈ«2H2O
ЈЁ2Ј© NaOHЈ¬ ЈЁ3Ј© KЈѕNaЈѕMgЈ¬
ЈЁ4Ј© Al2O3+6H+="2" Al3++3H2O
ПИіцПЦ°ЧЙ«іБµнЈ¬ІўЦрЅҐФц¶а AlCl3+3NH3Ў¤ H2O=Al(OH)3Ўэ+ 3NH4ClЈ¬
ЈЁ5Ј©CЈ«4HNO3(ЕЁ)
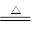 CO2ЎьЈ«4NO2ЎьЈ«2H2O
CO2ЎьЈ«4NO2ЎьЈ«2H2OїјІйФЄЛШЦЬЖЪ±нµДЅб№№єНФЄЛШЦЬЖЪВЙµДУ¦УГЎЈёщѕЭФЄЛШФЪЦЬЖЪ±нЦРµДО»ЦГїЙЦЄЈ¬ўЩЎ«?·Ц±рКЗNaЎўKЎўMgЎўCaЎўAlЎўCЎўOЎўClЎўBrЎўArЎўFЎЈ
ЈЁ1Ј©ПЎУРЖшМеФЄЛШµДЧоНвІгµзЧУКэТСѕґпµЅОИ¶ЁЅб№№Ј¬ЛщТФПЎУРЖшМеФЄЛШµДРФЦКЧоОИ¶ЁЈ¬јґКЗArЎЈ·ЗЅрКфРФЧоЗїµДКЗFЎЈЅрКфРФЧоЗїµДКЗјШЈ¬єНЛ®·ґУ¦µД·ЅіМКЅОЄK+2H2O=2K++2OH-+H2ЎьЎЈ
ЈЁ2Ј©ЅрКфРФФЅЗїЈ¬ЧоёЯјЫСх»ЇОпµДЛ®»ЇОпµДјоРФФЅЗїЈ¬ЅрКфРФКЗNaЈѕMgЈѕAlЈ¬ЛщТФЗвСх»ЇДЖµДјоРФЧоЗїЎЈ
ЈЁ3Ј©Н¬ЦЬЖЪЧФЧуПтУТФЧУ°лѕ¶ЦрЅҐјхРЎЈ¬Н¬ЦчЧеЧФЙП¶шПВФЧУ°лѕ¶ЦрЅҐФцґуЈ¬ЛщТФЛіРтКЗKЈѕNaЈѕMgЎЈ
ЈЁ4Ј©ЗвСх»ЇВБКЗБЅРФЗвСх»ЇОпЈ¬јИДЬУлЛб·ґУ¦ЙъіЙСОєНЛ®Ј¬УЦДЬєНјо·ґУ¦ЙъіЙСОєНЛ®Ј¬ЛщТФєНСОЛб·ґУ¦µДАлЧУ·ЅіМКЅОЄAl2O3+6H+="2" Al3++3H2OЎЈВИ»ЇВБєН°±Л®·ґУ¦ЙъіЙЗвСх»ЇВБ°ЧЙ«іБµнЈ¬µ«ЗвСх»ЇВБІ»ДЬИЬУЪ°±Л®ЦРЈ¬ЛщТФіБµнІ»»бПыК§ЎЈ·ЅіМКЅОЄAlCl3+3NH3Ў¤ H2O=Al(OH)3Ўэ+ 3NH4ClЎЈ
ЈЁ5Ј©ФЪјУИИµДМхјюПВЈ¬МјєНЕЁПхЛб·ґУ¦ЙъіЙCO2ЎўNO2єНЛ®Ј¬·ЅіМКЅОЄCЈ«4HNO3(ЕЁ)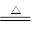 CO2ЎьЈ«4NO2ЎьЈ«2H2OЎЈ
CO2ЎьЈ«4NO2ЎьЈ«2H2OЎЈ
ЈЁ1Ј©ПЎУРЖшМеФЄЛШµДЧоНвІгµзЧУКэТСѕґпµЅОИ¶ЁЅб№№Ј¬ЛщТФПЎУРЖшМеФЄЛШµДРФЦКЧоОИ¶ЁЈ¬јґКЗArЎЈ·ЗЅрКфРФЧоЗїµДКЗFЎЈЅрКфРФЧоЗїµДКЗјШЈ¬єНЛ®·ґУ¦µД·ЅіМКЅОЄK+2H2O=2K++2OH-+H2ЎьЎЈ
ЈЁ2Ј©ЅрКфРФФЅЗїЈ¬ЧоёЯјЫСх»ЇОпµДЛ®»ЇОпµДјоРФФЅЗїЈ¬ЅрКфРФКЗNaЈѕMgЈѕAlЈ¬ЛщТФЗвСх»ЇДЖµДјоРФЧоЗїЎЈ
ЈЁ3Ј©Н¬ЦЬЖЪЧФЧуПтУТФЧУ°лѕ¶ЦрЅҐјхРЎЈ¬Н¬ЦчЧеЧФЙП¶шПВФЧУ°лѕ¶ЦрЅҐФцґуЈ¬ЛщТФЛіРтКЗKЈѕNaЈѕMgЎЈ
ЈЁ4Ј©ЗвСх»ЇВБКЗБЅРФЗвСх»ЇОпЈ¬јИДЬУлЛб·ґУ¦ЙъіЙСОєНЛ®Ј¬УЦДЬєНјо·ґУ¦ЙъіЙСОєНЛ®Ј¬ЛщТФєНСОЛб·ґУ¦µДАлЧУ·ЅіМКЅОЄAl2O3+6H+="2" Al3++3H2OЎЈВИ»ЇВБєН°±Л®·ґУ¦ЙъіЙЗвСх»ЇВБ°ЧЙ«іБµнЈ¬µ«ЗвСх»ЇВБІ»ДЬИЬУЪ°±Л®ЦРЈ¬ЛщТФіБµнІ»»бПыК§ЎЈ·ЅіМКЅОЄAlCl3+3NH3Ў¤ H2O=Al(OH)3Ўэ+ 3NH4ClЎЈ
ЈЁ5Ј©ФЪјУИИµДМхјюПВЈ¬МјєНЕЁПхЛб·ґУ¦ЙъіЙCO2ЎўNO2єНЛ®Ј¬·ЅіМКЅОЄCЈ«4HNO3(ЕЁ)
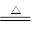 CO2ЎьЈ«4NO2ЎьЈ«2H2OЎЈ
CO2ЎьЈ«4NO2ЎьЈ«2H2OЎЈ
Б·П°ІбПµБРґр°ё
 ФД¶БїміµПµБРґр°ё
ФД¶БїміµПµБРґр°ё
Па№ШМвДї
 Y ўЪa+c
Y ўЪa+c
 HЈ«Ј«AЈ
HЈ«Ј«AЈ (HA)2
(HA)2 PtУл
PtУл PtµДЛµ·ЁХэИ·µДКЗ
PtµДЛµ·ЁХэИ·µДКЗ PtУл
PtУл PtµДЦКБїКэПаН¬
PtµДЦКБїКэПаН¬ PtУл
PtУл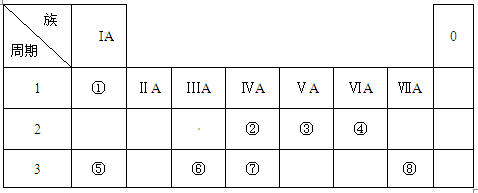
 ЈЁ2Ј©ўЪЎўўЫЎўўЯµДЧоёЯјЫє¬СхЛбµДЛбРФУЙЗїµЅИхµДЛіРтКЗ____________ЎЈ
ЈЁ2Ј©ўЪЎўўЫЎўўЯµДЧоёЯјЫє¬СхЛбµДЛбРФУЙЗїµЅИхµДЛіРтКЗ____________ЎЈ