��Ŀ����
��12�֣��±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
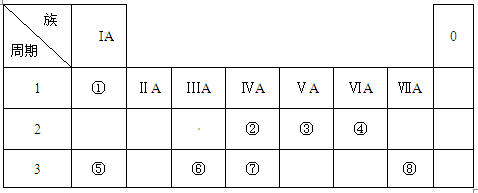
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ___________________��
 ��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����____________��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����____________��
 ��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________��
 ��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________��
��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________��
 a.MnO2 b.FeCl3 c.Na2SO3 d.KMnO4
a.MnO2 b.FeCl3 c.Na2SO3 d.KMnO4
 ��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
 X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ_____________________��
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ_____________________��
 N���ĵ��ʵĻ�ѧ����ʽΪ________________��
N���ĵ��ʵĻ�ѧ����ʽΪ________________��

��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ___________________��
 ��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����____________��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����____________�� ��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________�� ��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________��
��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________�� a.MnO2 b.FeCl3 c.Na2SO3 d.KMnO4
a.MnO2 b.FeCl3 c.Na2SO3 d.KMnO4 ��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
 X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ_____________________��
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ_____________________�� N���ĵ��ʵĻ�ѧ����ʽΪ________________��
N���ĵ��ʵĻ�ѧ����ʽΪ________________����1��Na��Al��O (2)HNO3��H2CO3��H2SiO3 (2)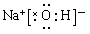 ��4��a b
��4��a b
��5��Al3����3NH3��H2O2=Al(OH)3����3NH4����2Al2O3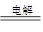 4Al��3O2��
4Al��3O2��
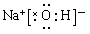 ��4��a b
��4��a b��5��Al3����3NH3��H2O2=Al(OH)3����3NH4����2Al2O3
 4Al��3O2��
4Al��3O2����1��ͬ������������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶���������Դ�С˳��ΪNa��Al��O��
��2���ǽ�����Խǿ������������ˮ���������Խǿ���ǽ�������N��C��Si����������˳����)HNO3��H2CO3��H2SiO3 ��
��3���١��ܡ��ݡ���ֱ���H��O��Na��Cl�����ԼȺ����Ӽ��ֺ����Թ��ۼ��Ļ��������������ƻ�������ƣ�����ʽ�ֱ���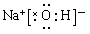 ��
��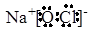 ��
��
��4��1��1��ɵij���Һ̬��������˫��ˮ��������a��b��
��2���ǽ�����Խǿ������������ˮ���������Խǿ���ǽ�������N��C��Si����������˳����)HNO3��H2CO3��H2SiO3 ��
��3���١��ܡ��ݡ���ֱ���H��O��Na��Cl�����ԼȺ����Ӽ��ֺ����Թ��ۼ��Ļ��������������ƻ�������ƣ�����ʽ�ֱ���
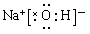 ��
��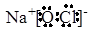 ��
����4��1��1��ɵij���Һ̬��������˫��ˮ��������a��b��

��ϰ��ϵ�д�
�����Ŀ
