��Ŀ����
(12��)A��B��C��D��E�Ǻ˵����������������ֶ���������Ԫ�أ�AԪ�ص�ԭ�Ӻ���ֻ��1�����ӣ�BԪ�ص�ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ����ࣴ����C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��C��E���壮
��1��B�����ڱ��е�λ�õ� ���ڣ��� �壻
��2��EԪ���γɵ��������Ӧ��ˮ����Ļ�ѧʽΪ ��
��3��Ԫ��C��D��E�γɵ����Ӱ뾶��С��ϵ�� �� �� �������ӷ��ű�ʾ����
��4��C��D���γɻ�����D2C2��D2C2���еĻ�ѧ���� ��
��5����A��B��C����Ԫ���γɵ����ӻ�����Ļ�ѧʽΪ ,
����ǿ����Һ��Ӧ�����ӷ���ʽ�� ��
(1)�� VA (2)H2SO4 (3)S2- >O2->Na+ (4)���ۼ� ���Ӽ�
(5)NH4NO3 NH4+ + OH- =NH3��H2O
����:

��ϰ��ϵ�д�
 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
�����Ŀ
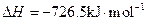 ��������ȼ����
��������ȼ����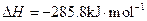 )
)