��Ŀ����
���淴Ӧ2NH3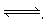 N2+3H2���ܱ������н��У��ﵽƽ��״̬�ı�־�ǣ� ��
N2+3H2���ܱ������н��У��ﵽƽ��״̬�ı�־�ǣ� ��
�ٵ�λʱ��������n mol N2��ͬʱ����2n mol NH3 �ڵ�λʱ��������n mol N2��ͬʱ����3n mol H2 ����NH3��N2��H2�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����֮��Ϊ2��1��3 �ܸ������Ũ�Ȳ��ٸı� �ݻ�������ƽ����Է����������ٸı�?
 N2+3H2���ܱ������н��У��ﵽƽ��״̬�ı�־�ǣ� ��
N2+3H2���ܱ������н��У��ﵽƽ��״̬�ı�־�ǣ� ���ٵ�λʱ��������n mol N2��ͬʱ����2n mol NH3 �ڵ�λʱ��������n mol N2��ͬʱ����3n mol H2 ����NH3��N2��H2�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����֮��Ϊ2��1��3 �ܸ������Ũ�Ȳ��ٸı� �ݻ�������ƽ����Է����������ٸı�?
| A���٢ܢ� | B���ڢۢ�? | C���٢ۢ� | D���٢ڢۢܢ�? |
A
���淴Ӧ2NH3 N2+3H2���ߵ�����ϵ������ȣ��ڴﵽƽ��ʱ�������ʵ�Ũ�ȡ������ʵ�����ƽ����Է����������ٸı䣬����Ϊƽ���жϵı���ƽ��״̬�ı��������淴Ӧ������ȣ��ٷ������⣬����ֻ����������Ӧ���ʣ��ų������Ƿ�Ӧ������һ������״̬�������ܱ����ﵽƽ��״̬��
N2+3H2���ߵ�����ϵ������ȣ��ڴﵽƽ��ʱ�������ʵ�Ũ�ȡ������ʵ�����ƽ����Է����������ٸı䣬����Ϊƽ���жϵı���ƽ��״̬�ı��������淴Ӧ������ȣ��ٷ������⣬����ֻ����������Ӧ���ʣ��ų������Ƿ�Ӧ������һ������״̬�������ܱ����ﵽƽ��״̬��
 N2+3H2���ߵ�����ϵ������ȣ��ڴﵽƽ��ʱ�������ʵ�Ũ�ȡ������ʵ�����ƽ����Է����������ٸı䣬����Ϊƽ���жϵı���ƽ��״̬�ı��������淴Ӧ������ȣ��ٷ������⣬����ֻ����������Ӧ���ʣ��ų������Ƿ�Ӧ������һ������״̬�������ܱ����ﵽƽ��״̬��
N2+3H2���ߵ�����ϵ������ȣ��ڴﵽƽ��ʱ�������ʵ�Ũ�ȡ������ʵ�����ƽ����Է����������ٸı䣬����Ϊƽ���жϵı���ƽ��״̬�ı��������淴Ӧ������ȣ��ٷ������⣬����ֻ����������Ӧ���ʣ��ų������Ƿ�Ӧ������һ������״̬�������ܱ����ﵽƽ��״̬��
��ϰ��ϵ�д�
 Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д� ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
�����Ŀ
 cC(g)��dD(g)�ı������ﵽƽ���D��Ũ���������ı�Ĺ�ϵ����ͼ�����жϴ˷�Ӧ��ϵ����ϵ����H���� �� ��
cC(g)��dD(g)�ı������ﵽƽ���D��Ũ���������ı�Ĺ�ϵ����ͼ�����жϴ˷�Ӧ��ϵ����ϵ����H���� �� ��
 2Z ��H< 0�ﵽƽ��ʱ���������¶�����ʹ
2Z ��H< 0�ﵽƽ��ʱ���������¶�����ʹ
 X
X
 2C��g������2 s ����C��Ũ��Ϊ0.6 mol/L�����м���˵����ȷ����
2C��g������2 s ����C��Ũ��Ϊ0.6 mol/L�����м���˵����ȷ���� 2C(g)�ﵽƽ��ʱ��A��B��C�����ʵ����ֱ�Ϊ4mol��2mol��4mol�������¶Ⱥ�������䣬��ƽ�����������ߵ����ʵ��������µ�������ʹƽ�����Ƶ���( )
2C(g)�ﵽƽ��ʱ��A��B��C�����ʵ����ֱ�Ϊ4mol��2mol��4mol�������¶Ⱥ�������䣬��ƽ�����������ߵ����ʵ��������µ�������ʹƽ�����Ƶ���( ) 2NH3(g)����H<0
2NH3(g)����H<0 2SO2(g)+O2(g)����H>0
2SO2(g)+O2(g)����H>0 2NO2(g)������˵���÷�Ӧ�Ѵﵽƽ��״̬����( ) ��
2NO2(g)������˵���÷�Ӧ�Ѵﵽƽ��״̬����( ) ��