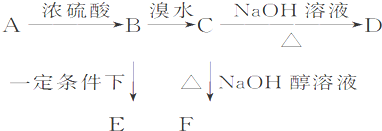��Ŀ����
��֪�л���A��B��C��D��E��F������ת����ϵ��A�IJ����Ǻ���һ������ʯ�ͻ�������ˮƽ�ı�־��D��ʹʯ����Һ��죻E�Dz�����ˮ�Ҿ�����ζ����ɫҺ�壬��Է���������C��2����F�Ǹ߷��Ӿۺ����������ʳƷ��װ���������ͼ��ϵ�ش����⣺
�Ű�Ҫ��ش��������⣺
��д��A��C�Ľṹ��ʽ��A ��C ��
��д��B��D�й����ŵ����ƣ�B ��D ��
��д����Ӧ�ڵķ�Ӧ����ʽ��
�����й���C��һ��ͬϵ���ȩ��˵������ȷ����__________������ĸ����
a����ȩ�Ľṹ��ʽΪHCHO��������Ϊ��CHO
b����ȩ��ȫȼ�պ�IJ���ΪCO2��H2O
c����ȩ��������������ͬ�Ĺ����ţ�����Һ���������Ʊ���Cu(OH)2����Һ��Ӧ
d����ȩ��ˮ��Һ����ɱ�����������ã����Դ�������ʳƷ�ӹ�ҵ
��A�뱽����ʯ�ͻ�������Ҫ��Ʒ����һ��������A����ת�����ɱ�����Ҫ��ش��������⣺
�ٱ����Է���ȡ����Ӧ��д���ɱ��Ʊ��屽�Ļ�ѧ��Ӧ����ʽ��
�ڴ������屽����ɫ��״Һ�壬ʵ�����ƵõĴ��屽ͨ�����ܽ���Br2�ʺ�ɫ�����Լ����Լ� ��ȥ����Ӧ����ʽΪ ���ó��Ӳ���������IJ��������� ��
��13�֣��Ţ�CH2=CH2��CH3CHO �����ǻ����Ȼ� ����2CH3CH2OH +O2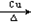 2CH3CHO +2H2O
2CH3CHO +2H2O
��d �Ǣ�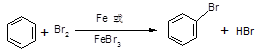
��NaOH��Һ ��Br2 + 2NaOH=NaBr+NaBrO+H2O����Һ©��
�������������A�IJ����Ǻ���һ������ʯ�ͻ�������ˮƽ�ı�־����A����ϩ����ϩ����̼̼˫�����ܺ�ˮ�����ӳɷ�Ӧ�����Ҵ�����B���Ҵ����Ҵ�����������������ȩ����C����ȩ��D��ʹʯ����Һ��죬���ܺ��Ҵ���Ӧ���ɵ�E�Dz�����ˮ�Ҿ�����ζ����ɫҺ�壬��Է���������C��2������˵��D�����ᣬE������������F�Ǹ߷��Ӿۺ����������ʳƷ��װ��������F�Ǿ���ϩ���������ױ�����������Һ���գ�������Ҫ�����屽�е�Br2�ʳ�ȥ�����Լ����Լ�������������Һ�������屽������ˮ����Һ����ʵ�ַ��롣
���㣺�����л���ĺϳɺ��Ʊ������ʵķ�����ᴿ�Լ�������ѧ�������д
�������������е��Ѷȵ����⣬������۽̲Ļ���֪ʶ��ּ�ڹ���ѧ���Ļ��������ѧ��������û���֪ʶ���ʵ�����������������������ѧ�������������������ѧ����Ӧ������������Ĺؼ�����ͻ�Ƶ��Լ������л���Ľṹ�����ʣ�Ȼ��������ü��ɡ�