��Ŀ����
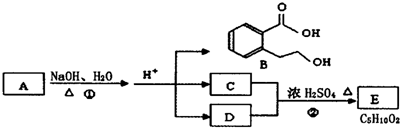
��֪�л��� A��B��C��D��E��F֮���ת����ϵ��ͼ��ʾ��D �dz����������������Ŀ�������EΪ�ۺ��F��ʽ��Ϊ26��������Ϣ�ش��������⣮
��1��д��A��F�Ľṹ��ʽ��A
��2��д�����з�Ӧ�Ļ�ѧ����ʽ����Ӧ���ͣ�Bת��ΪE�ķ�Ӧ��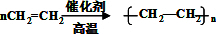
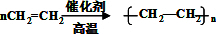 ����Ӧ����
����Ӧ����
Cת��ΪD�ķ�Ӧ��

��1��д��A��F�Ľṹ��ʽ��A
CH3CH2OH
CH3CH2OH
��FCH��CH
CH��CH
��A�й����������ǻ�
�ǻ�
����2��д�����з�Ӧ�Ļ�ѧ����ʽ����Ӧ���ͣ�Bת��ΪE�ķ�Ӧ��
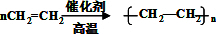
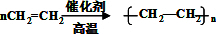
�ۺϷ�Ӧ���Ӿ۷�Ӧ��
�ۺϷ�Ӧ���Ӿ۷�Ӧ��
Cת��ΪD�ķ�Ӧ��
CH2BrCH2Br+2H2O
CH2OHCH2OH+2HBr
| NaOH |
CH2BrCH2Br+2H2O
CH2OHCH2OH+2HBr
����Ӧ����| NaOH |
ˮ�⣨��ȡ����
ˮ�⣨��ȡ����
��������D�dz����������������Ŀ�������ӦΪCH2OHCH2OH����CӦΪCH2BrCH2Br��CH2BrCH2Br������ȥ��Ӧ����CH��CH��ʽ��Ϊ26��EΪ�ۺ����B�ӳɿ�����C��˵��BӦΪCH2=CH2��A��Ũ���������¼�����1700C�����ɣ�˵��AΪCH3CH2OH��������ʵĽṹ�����ʽ����⣮
����⣺D�dz����������������Ŀ�������ӦΪCH2OHCH2OH����CӦΪCH2BrCH2Br��CH2BrCH2Br������ȥ��Ӧ����CH��CH��ʽ��Ϊ26��EΪ�ۺ����B�ӳɿ�����C��˵��BӦΪCH2=CH2��A��Ũ���������¼�����1700C�����ɣ�˵��AΪCH3CH2OH����
��1�������Ϸ�����֪AΪCH3CH2OH�����еĹ�����Ϊ�ǻ���FΪCH��CH���ʴ�Ϊ��CH3CH2OH��CH��CH���ǻ���
��2��BΪCH2=CH2����һ�������¿ɷ����Ӿ۷�Ӧ���ɾ���ϩ����Ӧ�ķ���ʽΪ��
�ʴ�Ϊ��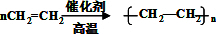 ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ��
��3��CΪCH2BrCH2Br����NaOH��ˮ��Һ�з���ˮ�⣨��ȡ��������CH2OHCH2OH�������������������Ŀ���������Ӧ�ķ���ʽΪCH2BrCH2Br+2H2O
CH2OHCH2OH+2HBr��
�ʴ�Ϊ��CH2BrCH2Br+2H2O
CH2OHCH2OH+2HBr��ˮ�⣨��ȡ������
��1�������Ϸ�����֪AΪCH3CH2OH�����еĹ�����Ϊ�ǻ���FΪCH��CH���ʴ�Ϊ��CH3CH2OH��CH��CH���ǻ���
��2��BΪCH2=CH2����һ�������¿ɷ����Ӿ۷�Ӧ���ɾ���ϩ����Ӧ�ķ���ʽΪ��
�ʴ�Ϊ��
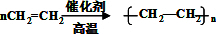 ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ����3��CΪCH2BrCH2Br����NaOH��ˮ��Һ�з���ˮ�⣨��ȡ��������CH2OHCH2OH�������������������Ŀ���������Ӧ�ķ���ʽΪCH2BrCH2Br+2H2O
| NaOH |
�ʴ�Ϊ��CH2BrCH2Br+2H2O
| NaOH |
���������⿼���л�����ƶϣ���Ŀ�ѶȲ�����ע���л������Ҫ��;���ڸ�����Ϊ������Ĺؼ�����CH2OHCH2OH�����������������Ŀ�����������ע���л���Ĺ����ź����ʣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ