��Ŀ����
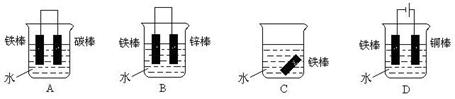
��6�֣�������ҵ�ǹ��ҹ�ҵ�Ļ�������ش����ұ������ʴ�����������е��й����⡣
(1)��ҵ���Ȼ�ԭ��������д����CO��ԭ������(��Ҫ�ɷ�ΪFe2O3)�Ļ�ѧ����ʽ��____ ______________��
(2)���ڳ�ʪ�Ŀ��������ױ���ʴΪ����(Fe2O3��xH2O)����Ӧ�Ļ�ѧ����ʽΪ_____________ ___ _________��
(3)�����п��������������⡣�ֽ�һ�������Ƭ���������У������ⱻ��ȫ��������Һ�м��������Ļ��Ϸ�Ӧ�Ļ�ѧ����ʽΪ____________________________��
(1)��ҵ���Ȼ�ԭ��������д����CO��ԭ������(��Ҫ�ɷ�ΪFe2O3)�Ļ�ѧ����ʽ��____ ______________��
(2)���ڳ�ʪ�Ŀ��������ױ���ʴΪ����(Fe2O3��xH2O)����Ӧ�Ļ�ѧ����ʽΪ_____________ ___ _________��
(3)�����п��������������⡣�ֽ�һ�������Ƭ���������У������ⱻ��ȫ��������Һ�м��������Ļ��Ϸ�Ӧ�Ļ�ѧ����ʽΪ____________________________��
��6�֣���1)3CO��Fe2O32Fe��3CO2
(2)4Fe��3O2��2xH2O��2Fe2O3��xH2O(������������) (3)2FeCl3��Fe��3FeCl2
(2)4Fe��3O2��2xH2O��2Fe2O3��xH2O(������������) (3)2FeCl3��Fe��3FeCl2
��

��ϰ��ϵ�д�
 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ
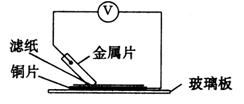
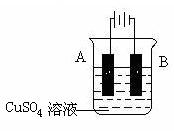
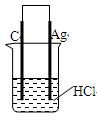
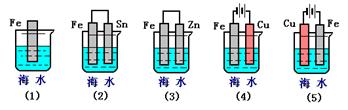
 ������ֽ���ܿ�������ɫ���������Ľ�����__________(����ĸ)�����Ӧ��صĵ缫��ӦʽΪ��
������ֽ���ܿ�������ɫ���������Ľ�����__________(����ĸ)�����Ӧ��صĵ缫��ӦʽΪ��