��Ŀ����
����ѧ�����ʽṹ�����ʡ���15�֣�
�±���ʵ����Ԫ�����ڱ��IJ��ֱ߽磬�����ϱ߽粢δ��ʵ�߱����
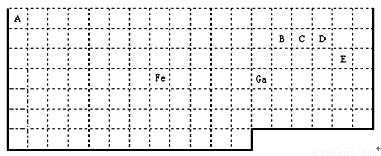
������Ϣ�ش��������⣺
��1�����ڱ��б�Ga��������2�Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ ��
��2��FeԪ��λ�����ڱ��� ������Fe��CO���γ������Fe(CO)5����Fe(CO)5�����Ļ��ϼ�Ϊ__________����֪��ԭ����Ŀ�͵�����������۵�����������ͬ������Ϊ�ȵ����壬�ȵ�����������ƵĽṹ��������CO���ӻ�Ϊ�ȵ�����ķ��Ӻ����ӷֱ�Ϊ��������____��������_____���ѧʽ����CO�ĽṹʽΪ�� ��
��3����CH4��CO��CH3OH�У�̼ԭ�Ӳ�ȡsp3�ӻ��ķ���Ϊ��������������������
��4������VSEPR����Ԥ��ED4- ���ӵĿռ乹��Ϊ______________�͡�B��C��D��Eԭ��������γɵķ����У�����ԭ�Ӷ����������8�����ȶ��ṹ�ĵ���ʽΪ��__________________________________(д2��) ��
��5��B��D�γɵ��ȶ�������Ϊ___________���ӣ�����ԡ����Ǽ��ԡ��������̬Ϊ ________���塣
��1��3d104s1 ��3�� ��
��2��d 0 N2 CN�� C��O (ÿ��1�֣���5��)
��3��CH4 CH3OH ��ÿ��1�֣���2�֣�
��4���������壻
����ΪCO2��NCl3��CCl4��CO�ȵ���ʽ�е�����2�� ��ÿ��1�֣���3�֣�
��5���Ǽ��� ���� ��ÿ��1�֣���2�֣�
��������

 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� ��2011?����ģ�⣩����ѧ--���ʽṹ�����ʡ�
��2011?����ģ�⣩����ѧ--���ʽṹ�����ʡ�
 ��2010?���϶�ģ������ѧ-���ʽṹ�����ʡ�
��2010?���϶�ģ������ѧ-���ʽṹ�����ʡ� ����ѧ-���ʽṹ�����ʡ�
����ѧ-���ʽṹ�����ʡ� ����ѧ--���ʽṹ�����ʡ�
����ѧ--���ʽṹ�����ʡ�