��Ŀ����
�����йؽ�����ȷ���� �� ��
A��T��ʱ��Ba��OH��2��Һ��c��H+����c��OH-��=10-12����pH=8�ĸ���Һ�м�������pH=4�����ᣬ������Һ��pH=7
B��pH��ȵ����ᣮ���ᣮ���ᣬ��ϡ��1000����ϡ�ͺ�pH�����Ǵ���
C��0��2mol/L��HA��Һ��0��1mol/L��NaOH��Һ�������ϣ����Һ�ڳ���ʱpHһ��С��7
D����0��1mol/L�����������Һ�и�����Ũ�ȹ�ϵ�ǣ�c��H+�� ��c��SO42���� �� c��NH4���� �� c��OH-��
D

��ϰ��ϵ�д�
�����Ŀ
�����йؽ�����ȷ���ǣ�������
| A��T��ʱ��Ba��OH��2��Һ��c��H+��?c��OH-��=10-12����pH=8�ĸ���Һ��������pH=4�����ᣬ����ҺpH=7 | B��pH��ȵ����ᡢ���ᡢ���ᣬ��ϡ��1000����ϡ�ͺ�pH�����Ǵ��� | C��0.2mol/L��HA��Һ��0.1mol/L��NaOH��Һ�������ϣ����Һ�ڳ���ʱpHһ��С��7 | D����0.1mol/L�����������Һ�и�����Ũ�ȹ�ϵ�ǣ�c(H+)��c(SO42-)��c(NH4+)��c(OH-) |
�����йؽ�����ȷ����
| A��T��ʱ��Ba��OH��2��Һ��c��H+����c��OH-��=10-12����pH=8�ĸ���Һ��������pH=4�����ᣬ����ҺpH=7 |
| B��pH��ȵ����ᡢ���ᡢ���ᣬ��ϡ��1000����ϡ�ͺ�pH�����Ǵ��� |
| C��0.2mol��L��HA��Һ��0.1mol��L��NaOH��Һ�������ϣ����Һ�ڳ���ʱpHһ��С��7 |
| D����0.1mol��L�����������Һ�и�����Ũ�ȹ�ϵ�ǣ�c(H+)>c(SO42-)>c(NH4+)>c(OH-) |
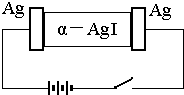 ��-AgI��һ�ֹ��嵼�壬�����ʺܸߣ�Ϊ�о� ��-AgI ������ Ag+���绹�� I-���磬�������ͼʵ�飬�����йؽ�����ȷ���ǣ�������
��-AgI��һ�ֹ��嵼�壬�����ʺܸߣ�Ϊ�о� ��-AgI ������ Ag+���绹�� I-���磬�������ͼʵ�飬�����йؽ�����ȷ���ǣ�������