��Ŀ����
������dz�����θȳ�������Ǽס������ֳ���θҩ��˵��ժҪ
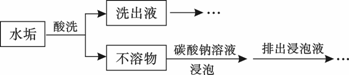
�ף���1����ɫ�ᾧ״��ĩ���ɻ����ֽ⣻��2��������ˮ��ˮ��Һ�������ԣ���3�����ἰ����ҩ�������������̼����4��θ�����߷��ö��¶����������������θ����Σ�գ�
�ң���1����θ�����к����û������־ã���ά��3��4Сʱ����2�����������������������ϣ����б������ã���3��������ϡ�������������Һ�У�
��1�������Ʋ⣬���к��е���Ҫ��ѧ�ɷ���______���ѧʽ�������к��е���Ҫ��ѧ�ɷ���______�����ѧʽ����
��2����д�����к��е���Ҫ��ѧ�ɷ�����θ������Ӧ�����ӷ���ʽ��______��
��3����д�����к��е���Ҫ��ѧ�ɷ�������������Һ��Ӧ�����ӷ���ʽ��______��
�ף���1����ɫ�ᾧ״��ĩ���ɻ����ֽ⣻��2��������ˮ��ˮ��Һ�������ԣ���3�����ἰ����ҩ�������������̼����4��θ�����߷��ö��¶����������������θ����Σ�գ�
�ң���1����θ�����к����û������־ã���ά��3��4Сʱ����2�����������������������ϣ����б������ã���3��������ϡ�������������Һ�У�
��1�������Ʋ⣬���к��е���Ҫ��ѧ�ɷ���______���ѧʽ�������к��е���Ҫ��ѧ�ɷ���______�����ѧʽ����
��2����д�����к��е���Ҫ��ѧ�ɷ�����θ������Ӧ�����ӷ���ʽ��______��
��3����д�����к��е���Ҫ��ѧ�ɷ�������������Һ��Ӧ�����ӷ���ʽ��______��
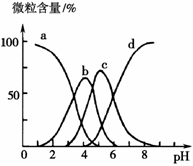
��1������Ϣ��֪���������ֽܷ⣬ˮ���Լ��ԣ����ᷴӦ���ɶ�����̼���������Ҫ����Ϊ̼�����ƣ��仯ѧʽΪNaHCO3���������ʿ�����ϡ�������������Һ�����������������������ϣ����б������ã������к��е���Ҫ����Ϊ�����������仯ѧʽΪAl��OH��3���ʴ�Ϊ��NaHCO3��Al��OH��3��
��2������̼�����������ᷴӦ�����Ȼ��ơ�ˮ��������̼���÷�Ӧ�����ӷ���ʽΪHCO3-+H+�TCO2��+H2O���ʴ�Ϊ��HCO3-+H+�TCO2��+H2O��
��3����������������������Һ��Ӧ����ƫ�����ƺ�ˮ���÷�Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��2������̼�����������ᷴӦ�����Ȼ��ơ�ˮ��������̼���÷�Ӧ�����ӷ���ʽΪHCO3-+H+�TCO2��+H2O���ʴ�Ϊ��HCO3-+H+�TCO2��+H2O��
��3����������������������Һ��Ӧ����ƫ�����ƺ�ˮ���÷�Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��

��ϰ��ϵ�д�
 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
�����Ŀ