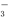��Ŀ����
���ں���Na�� ��Mg2����HCO3����SO42����1Lϡ��Һ�У����ǵ����ʵ���Ϊ2mol,1mol,2mol,1mol��
��Mg2����HCO3����SO42����1Lϡ��Һ�У����ǵ����ʵ���Ϊ2mol,1mol,2mol,1mol��
�������Һ�еμӺ�0.5mol�������Ƶ�100mL��Һ�йط�Ӧ�����ӷ���ʽΪ��
�������Һ�еμӺ�1.5mol�������Ƶ�100mL��Һ�йط�Ӧ�����ӷ���ʽΪ��
�������Һ�еμӺ�3mol����������100mL��Һ�йط�Ӧ�����ӷ���ʽΪ��
��ij�������Һ�п��ܴ��ڵ�����Ϊ��Ag+��H+��Cu2+��CO32-��OH����Cl������Ϊ���Կ϶����ڵ������У� ���϶�û�е������ǣ� �������һ��ȷ�ϵ������У� ȷ�ϵ�ʵ�鷽���ǣ� ��
��ϰ��ϵ�д�
 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
�����Ŀ


 ȡ����ֻ��3��
ȡ����ֻ��3��
 ����ʽ������������
����ʽ������������
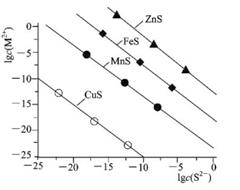
 ij����A��һ�������¼��ȷֽ⣬���ﶼ�����壬�ֽⷽ��ʽΪ2A
ij����A��һ�������¼��ȷֽ⣬���ﶼ�����壬�ֽⷽ��ʽΪ2A B+2C+2D��������ɵĻ�����������������ܶ�Ϊd����A����Է�������Ϊ
B+2C+2D��������ɵĻ�����������������ܶ�Ϊd����A����Է�������Ϊ Cu2����H2��
Cu2����H2��