��Ŀ����
��2009?��ƽ��ģ��A��B��C��DΪ���ֶ�����Ԫ�أ�A��B��D��ԭ��������ԭ�Ӱ뾶����������B��Dͬ�����������һ�����γɡ����ꡱ�Ļ����A��B�����γ�A2B��A2B2������ͨ������³�Һ̬�Ĺ��ۻ����B��C�γɵ��������ӻ���������ˮ�����õ���Һ����ǿ���ԣ�C�ĵ��ʳ����¿���A2B���ҷ�Ӧ���Իش��������⣺
��1��B��C��Ԫ������������1��1�γɵĻ�����X�У����������Ӹ�����Ϊ
��2����A2B2�����£�ͭ��ϡ����������ͭ�Ļ�ѧ��Ӧ�Ļ�ѧ����Ϊ��
��3����ʾ�γ�DB2�͡����ꡱ�Ļ�ѧ��Ӧ����ʽ�ж��������ѡ��һ�����ʵķ�Ӧ��д�������Ӧ��ƽ�ⳣ������ʽK=
��
��4����֪������ 17gA��D��Ԫ����ɵĻ�������������DB2��ȫ��Ӧʱ�ų�����Ϊa kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ
��5����֪ 25��ʱ��Ksp��CaCO3��=1��10-9��Ksp��CaSO4��=9��10-6������ʹ�õĹ�¯��Ҫ���ڳ�ˮ��������ή��ȼ�ϵ������ʣ�ˮ���к��е�CaSO4��������Na2CO3��Һ������ʹ֮ת��Ϊ���ɡ����������CaCO3�����������ȥ��
��CaSO4ת��ΪCaCO3�����ӷ���ʽΪ
�������CaSO4ת��ΪCaCO3��ԭ��
��1��B��C��Ԫ������������1��1�γɵĻ�����X�У����������Ӹ�����Ϊ
1��2
1��2
����2����A2B2�����£�ͭ��ϡ����������ͭ�Ļ�ѧ��Ӧ�Ļ�ѧ����Ϊ��
Cu+H2SO4+H2O2=CuSO4+2H2O
Cu+H2SO4+H2O2=CuSO4+2H2O
����3����ʾ�γ�DB2�͡����ꡱ�Ļ�ѧ��Ӧ����ʽ�ж��������ѡ��һ�����ʵķ�Ӧ��д�������Ӧ��ƽ�ⳣ������ʽK=
| c2(SO3) |
| c2(SO2)?c(O2) |
| c2(SO3) |
| c2(SO2)?c(O2) |
��4����֪������ 17gA��D��Ԫ����ɵĻ�������������DB2��ȫ��Ӧʱ�ų�����Ϊa kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ
2H2S��g��+SO2��g���T3S��s��+2H2O��l����H=-4akJ/mol
2H2S��g��+SO2��g���T3S��s��+2H2O��l����H=-4akJ/mol
����5����֪ 25��ʱ��Ksp��CaCO3��=1��10-9��Ksp��CaSO4��=9��10-6������ʹ�õĹ�¯��Ҫ���ڳ�ˮ��������ή��ȼ�ϵ������ʣ�ˮ���к��е�CaSO4��������Na2CO3��Һ������ʹ֮ת��Ϊ���ɡ����������CaCO3�����������ȥ��
��CaSO4ת��ΪCaCO3�����ӷ���ʽΪ
CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq��
CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq��
���������CaSO4ת��ΪCaCO3��ԭ��
����Na2CO3��Һ��CO32-��Ca2+��ϣ�ת��ΪKsp��С��CaCO3������ʹCaSO4�ij����ܽ�ƽ�����ܽⷽ���ƶ�
����Na2CO3��Һ��CO32-��Ca2+��ϣ�ת��ΪKsp��С��CaCO3������ʹCaSO4�ij����ܽ�ƽ�����ܽⷽ���ƶ�
��������B��Dͬ�����������һ�����γɡ����ꡱ�Ļ����������ΪSO2������ԭ��������ϵ��֪BΪOԪ�أ�DΪSԪ�أ�A��B�����γ�A2B��A2B2������ͨ������³�Һ̬�Ĺ��ۻ����ӦΪH2O��H2O2����AΪHԪ�أ�B��C�γɵ��������ӻ���������ˮ�����õ���Һ����ǿ���ԣ�C�ĵ��ʳ����¿���A2B���ҷ�Ӧ����C��H2O���ҷ�Ӧ���������ӻ�����ֱ���Na2O��Na2O2����CΪNaԪ�أ�
��1������B��C��Ԫ������������1��1�γɵĻ�����ΪNa2O2������
��2��Cu��ϡ�����Ӧ���ھ���ǿ�����Ե�H2O2�����£�������Ӧ����CuSO4��
��3���Է�Ӧ2SO2+O2 2SO3Ϊ��������ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮���жϣ�
2SO3Ϊ��������ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮���жϣ�
��4�����ݷ�Ӧ�Ļ�ѧ����ʽΪ2H2S+SO2�T3S+2H2O��������ʵ������㣻
��5���ӳ�����ת���ĽǶȷ�����
��1������B��C��Ԫ������������1��1�γɵĻ�����ΪNa2O2������
��2��Cu��ϡ�����Ӧ���ھ���ǿ�����Ե�H2O2�����£�������Ӧ����CuSO4��
��3���Է�Ӧ2SO2+O2
 2SO3Ϊ��������ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮���жϣ�
2SO3Ϊ��������ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮���жϣ���4�����ݷ�Ӧ�Ļ�ѧ����ʽΪ2H2S+SO2�T3S+2H2O��������ʵ������㣻
��5���ӳ�����ת���ĽǶȷ�����
����⣺B��Dͬ�����������һ�����γɡ����ꡱ�Ļ����������ΪSO2������ԭ��������ϵ��֪BΪOԪ�أ�DΪSԪ�أ�A��B�����γ�A2B��A2B2������ͨ������³�Һ̬�Ĺ��ۻ����ӦΪH2O��H2O2����AΪHԪ�أ�B��C�γɵ��������ӻ���������ˮ�����õ���Һ����ǿ���ԣ�C�ĵ��ʳ����¿���A2B���ҷ�Ӧ����C��H2O���ҷ�Ӧ���������ӻ�����ֱ���Na2O��Na2O2����CΪNaԪ�أ���
��1��B��C��Ԫ������������1��1�γɵĻ�����ΪNa2O2������������ΪNa+��������ΪO22-�����������Ӹ�����Ϊ1��2���ʴ�Ϊ��1��2��
��2��Cu��ϡ�����Ӧ���ھ���ǿ�����Ե�H2O2�����£�������Ӧ����CuSO4����Ӧ�Ļ�ѧ����ʽΪCu+H2SO4+H2O2=CuSO4+2H2O��
�ʴ�Ϊ��Cu+H2SO4+H2O2=CuSO4+2H2O��
��3���Է�Ӧ2SO2+O2 2SO3Ϊ������Ӧ��ƽ�ⳣ��Ϊk=
2SO3Ϊ������Ӧ��ƽ�ⳣ��Ϊk=
���ʴ�Ϊ��
��
��4��A��D��Ԫ����ɵĻ�������������DB2��ȫ��Ӧ�Ļ�ѧ����ʽΪ2H2S+SO2�T3S+2H2O��n��H2S��=
=0.5mol��
��4molH2S�μӷ�Ӧ����Һ̬ˮ�ų�������Ϊ4akJ��
�����Ȼ�ѧ����ʽΪ2H2S��g��+SO2��g���T3S��s��+2H2O��l����H=-4a kJ/mol��
�ʴ�Ϊ��2H2S��g��+SO2��g���T3S��s��+2H2O��l����H=-4a kJ/mol��
��5������Na2CO3��Һ��CO32-��Ca2+��ϣ�ת��ΪKsp��С��CaCO3������ʹCaSO4�ij����ܽ�ƽ�����ܽⷽ���ƶ�����Ӧ�����ӷ���ʽΪCaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq����CaCO3���ɡ��������ᣬ�Ӷ���ȥˮ����
�ʴ�Ϊ��CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq��������Na2CO3��Һ��CO32-��Ca2+��ϣ�ת��ΪKsp��С��CaCO3������ʹCaSO4�ij����ܽ�ƽ�����ܽⷽ���ƶ���
��1��B��C��Ԫ������������1��1�γɵĻ�����ΪNa2O2������������ΪNa+��������ΪO22-�����������Ӹ�����Ϊ1��2���ʴ�Ϊ��1��2��
��2��Cu��ϡ�����Ӧ���ھ���ǿ�����Ե�H2O2�����£�������Ӧ����CuSO4����Ӧ�Ļ�ѧ����ʽΪCu+H2SO4+H2O2=CuSO4+2H2O��
�ʴ�Ϊ��Cu+H2SO4+H2O2=CuSO4+2H2O��
��3���Է�Ӧ2SO2+O2
 2SO3Ϊ������Ӧ��ƽ�ⳣ��Ϊk=
2SO3Ϊ������Ӧ��ƽ�ⳣ��Ϊk=| c2(SO3) |
| c2(SO2)?c(O2) |
| c2(SO3) |
| c2(SO2)?c(O2) |
��4��A��D��Ԫ����ɵĻ�������������DB2��ȫ��Ӧ�Ļ�ѧ����ʽΪ2H2S+SO2�T3S+2H2O��n��H2S��=
| 17g |
| 34g/mol |
��4molH2S�μӷ�Ӧ����Һ̬ˮ�ų�������Ϊ4akJ��
�����Ȼ�ѧ����ʽΪ2H2S��g��+SO2��g���T3S��s��+2H2O��l����H=-4a kJ/mol��
�ʴ�Ϊ��2H2S��g��+SO2��g���T3S��s��+2H2O��l����H=-4a kJ/mol��
��5������Na2CO3��Һ��CO32-��Ca2+��ϣ�ת��ΪKsp��С��CaCO3������ʹCaSO4�ij����ܽ�ƽ�����ܽⷽ���ƶ�����Ӧ�����ӷ���ʽΪCaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq����CaCO3���ɡ��������ᣬ�Ӷ���ȥˮ����
�ʴ�Ϊ��CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq��������Na2CO3��Һ��CO32-��Ca2+��ϣ�ת��ΪKsp��С��CaCO3������ʹCaSO4�ij����ܽ�ƽ�����ܽⷽ���ƶ���
���������⿼���Ϊ�ۺϣ����Ԫ�ص��ƶϡ����ӵ���ɡ����ʵ������Լ��Ȼ�ѧ����ʽ�����⣬��Ŀ�Ѷ��еȣ��״���Ϊ��5����ע��ӳ���ת���ĽǶȷ�����

��ϰ��ϵ�д�
�����Ŀ
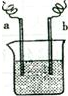 ��2009?��ƽ��ģ��ͼ�У����缫�Ϸ����ĵ缫��ӦΪ��a����Cu2++2e-=Cu b����Fe-2e-=Fe2+������˵���в���ȷ���ǣ�������
��2009?��ƽ��ģ��ͼ�У����缫�Ϸ����ĵ缫��ӦΪ��a����Cu2++2e-=Cu b����Fe-2e-=Fe2+������˵���в���ȷ���ǣ�������