��Ŀ����
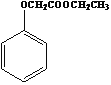
����Ŀ�������۵���ߵĽ���������Ҫ��ս�����ʣ���Ȼ�����ٿ�ʯ����Ҫ�ɷ��������̵�������(FeWO4��MnWO4)����������Si��P��As�Ļ�����ɺ��ٿ�ұ���ٵĹ���������ͼ��

��֪��������I����Ҫ�ɷ���Fe2O3��MnO2��
�����������У������һ���⣬���ಽ���ٵĻ��ϼ�δ�䣮
������������������ˮ��
�ش��������⣺
��1��������(FeWO4��MnWO4)����Ԫ�صĻ��ϼ�Ϊ__________����д��MnWO4�����������·�����ֽⷴӦ����MnO2�Ļ�ѧ����ʽ__________��
��2�����������������������Һ�м������pH=10����Һ�е�����������ȷSiO32-��HAsO32-��HAsO42-��HPO42-�ȣ����������������У�����H2O2ʱ������Ӧ�����ӷ���ʽΪ__________������������Ҫ�ɷ���__________��
��3����֪�������ƺ������(CaWO4)�����ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С��ͼΪ��ͬ�¶���Ca(OH)2��CaWO4�ij����ܽ�ƽ�����ߣ���T1ʱKsp(CaWO4)=__________molL-1������������Һ����ʯ����õ���������ƣ�������Ӧ�����ӷ���ʽΪ__________��T2ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ__________molL-1��
��4��Ӳ�ʺϽ��к�̼����(WC)�����õ�ⷨ���Դ�̼���ٷ����л����٣����ʱ����̼�������������������������HCl��ҺΪ���Һ�������������Ტ�ų�CO2����������ӦʽΪ__________��

���𰸡���1��+6��2MnWO4+O2+4NaOH![]() 2MnO2+2Na2WO4+2H2O��
2MnO2+2Na2WO4+2H2O��
��2��H2O2+HAsO32-�THAsO42-+H2O��MgSiO3��MgHAsO4��MgHPO4��
��3��1��10-10��WO42-+Ca(OH)2=CaWO4+2OH-��1��103����4��WC+6H2O-10e-=H2WO4+CO2��+10H+
��������
�����������1��������FeWO4Ϊ����������������(FeWO4��MnWO4)�������̵Ļ��ϼ۶�Ϊ+2������������(FeWO4��MnWO4)����Ԫ�صĻ��ϼ�Ϊ+x���������������ϼ۴�����Ϊ������+2+x+(-2)��4=0�����x=+6�����ٿ����Ҫ�ɷ��������̵�����������������ͼ����ʾ֪�����ٿ��ڿ�������������WO42-��MnO2����Fe2O3��������ת��������MnO2�Ļ�ѧ��Ӧ����ʽΪ2MnWO4+O2+4NaOH![]() 2MnO2+2Na2WO4+2H2O���ʴ�Ϊ��+6��2MnWO4+O2+4NaOH
2MnO2+2Na2WO4+2H2O���ʴ�Ϊ��+6��2MnWO4+O2+4NaOH![]() 2MnO2+2Na2WO4+2H2O��
2MnO2+2Na2WO4+2H2O��
��2���������Ϸ���������H2O2��Ŀ���ǽ�HAsO32-������HAsO42-�����ӷ���ʽΪH2O2+HAsO32-�THAsO42-+H2O����ҺI�д���SiO32-��HAsO32-��HAsO42-��HPO42-����������������pHֵ���������Ȼ�þ��Mg2+����SiO32-��HAsO32-��HAsO42-��HPO42-������������������Ҫ�ɷ���MgSiO3��MgHAsO4��MgHPO4���ʴ�Ϊ��H2O2+HAsO32-�THAsO42-+H2O��MgSiO3��MgHAsO4��MgHPO4��
��3��T1ʱKSP(CaWO4)=c(Ca2+)c(WO42-)=1��10-5��1��10-5=1��10-10������������Һ����ʯ���飬�������ֽⷴӦ���������ƺ���������ӷ�Ӧ��������Ƴ�������Ӧ�����ӷ���ʽΪ��WO42-+Ca(OH)2=CaWO4+2OH-��T2ʱ��C(OH-)=10-2mol/L��c(WO42-)=10-7mol/L��ƽ�ⳣ��K����������ƽ��Ũ��ϵ���η�֮���ͷ�Ӧ��ƽ��Ũ��ϵ���η�֮������K=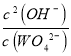 =
=![]() =1��103���ʴ�Ϊ��1��10-10��WO42-+Ca(OH)2=CaWO4+2OH-��1��103��
=1��103���ʴ�Ϊ��1��10-10��WO42-+Ca(OH)2=CaWO4+2OH-��1��103��
��4�����ʱ�������������ӷŵ������������缫��Ӧʽ��2H++2e-=H2����������̼����ʧȥ���ӣ�����������Ӧ��WC+6H2O-10e-=H2WO4+CO2��+10H+���ʴ�Ϊ��WC+6H2O-10e-=H2WO4+CO2��+10H+��

 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�