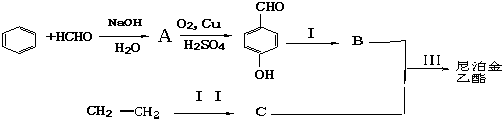
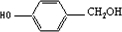
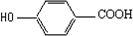
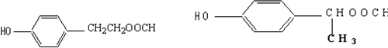��Ŀ����
2011��7��4�գ��㽭��ɽ����������60����������չ��Χ������۵ع���694�֣���ν���ع��͡��Ǵ�һЩ���ꡢ�Ƶ����ˮ�����̳����IJ���ʣ��������ġ���ˮ�͡��Լ���������ӹ��ɡ�ʳ���͡��������й�˵������ȷ���ǣ�������
| A�����ع��͡�����Ҫ�ɷ��ǵ����ʣ������н϶���Ԫ�� | B�����ع��͡�������ձ�ʳ����һ�������������ġ��ع��͡����й̶����۷е� | C�����ع��͡��ڼ������ݼ��������˶����彡����Ӱ�� | D���ع��Ϳ������������ |
������A�����ع��͡�����Ҫ�ɷ�����֬�������н϶����Ԫ�أ�
B�����ع��͡��ǻ���û�й̶����۷е㣻
C�����ع��͡��ڼ������ݼ�����������������彡����Ӱ�죻
D�����ع��͡��������õ��״�����һ��ת������������ͣ�
B�����ع��͡��ǻ���û�й̶����۷е㣻
C�����ع��͡��ڼ������ݼ�����������������彡����Ӱ�죻
D�����ع��͡��������õ��״�����һ��ת������������ͣ�
����⣺A�����ع��͡�����Ҫ�ɷ�����֬�������н϶����Ԫ�أ���A����
B�����ع��͡��ǻ���û�й̶����۷е㣬��B����
C�����ع��͡��ڼ������ݼ�����������������彡����Ӱ�죬��C����
D�����ع��͡��������õ��״�����һ��ת������������ͣ����ԡ��ع��͡�����������������ͣ���D��ȷ��
��ѡ��D��
B�����ع��͡��ǻ���û�й̶����۷е㣬��B����
C�����ع��͡��ڼ������ݼ�����������������彡����Ӱ�죬��C����
D�����ع��͡��������õ��״�����һ��ת������������ͣ����ԡ��ع��͡�����������������ͣ���D��ȷ��
��ѡ��D��
������������Ҫ�����˵ع��͵���Ҫ�ɷ���֬����ɡ��ṹ�����ʣ��Ѷ��еȣ�ע�����֪ʶ�Ļ������գ�

��ϰ��ϵ�д�
�����Ŀ