��Ŀ����
2011��12��30���й�����������ͨ���������ġ���������������������������Ⱦ����Ŀ����ֵ��������PM2.5ƽ��Ũ����ֵ���ս��˶�����������Ⱦ���Ũ����ֵ��
��1��������һ�����ȼ�ϣ���һ�������£�������Ӧ��CH4��g��+H2O��g��?CO��g��+3H2��g����H��0����1.0molCH4��2.0molH2Oͨ�뷴Ӧ�����������ݻ�Ϊ10L����10minĩ��0.10molCO���ɣ���10min�ڸ÷�Ӧ������v��H2��= ��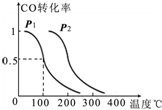
��2��ij��������β��CO�Ƽ״�����һ��ѹǿ�̶��ݻ��������У�ͨ��a molCO��2a molH2���ڴ��������·�Ӧ��CO��g��+2H2��g��?CH3OH��g����CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����
��P1 P2�����������������=������
���������������������£�������a molCO��2a molH2���ﵽ��ƽ��ʱ��CO��ת���� �����������С�����䡱����
��3��ij�Ƴ�Ϊ����CO2���ŷţ���ʯ��ˮ����CO2��25��ﵽ�����ܽ�ƽ��ʱ�������Һ��c��CO32-��=0.010mol?L-1����c��Ca2+�� ��д��������̣���֪��Ksp��CaCO3��=2.8��10-9����
��4��ij���᳧����β��NO2�����ǣ���������ʱ��H2��NO2��ԭΪN2��
��֪��2H2��g��+O2��g���T2H2O��g����H=-483.6 kJ/mol
N2��g��+2O2��g���T2NO2��g����H=+67.7 kJ/mol
��H2��ԭNO2����ˮ������Ӧ���Ȼ�ѧ����ʽ�� ��
��1��������һ�����ȼ�ϣ���һ�������£�������Ӧ��CH4��g��+H2O��g��?CO��g��+3H2��g����H��0����1.0molCH4��2.0molH2Oͨ�뷴Ӧ�����������ݻ�Ϊ10L����10minĩ��0.10molCO���ɣ���10min�ڸ÷�Ӧ������v��H2��=

��2��ij��������β��CO�Ƽ״�����һ��ѹǿ�̶��ݻ��������У�ͨ��a molCO��2a molH2���ڴ��������·�Ӧ��CO��g��+2H2��g��?CH3OH��g����CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����
��P1
���������������������£�������a molCO��2a molH2���ﵽ��ƽ��ʱ��CO��ת����
��3��ij�Ƴ�Ϊ����CO2���ŷţ���ʯ��ˮ����CO2��25��ﵽ�����ܽ�ƽ��ʱ�������Һ��c��CO32-��=0.010mol?L-1����c��Ca2+�� ��д��������̣���֪��Ksp��CaCO3��=2.8��10-9����
��4��ij���᳧����β��NO2�����ǣ���������ʱ��H2��NO2��ԭΪN2��
��֪��2H2��g��+O2��g���T2H2O��g����H=-483.6 kJ/mol
N2��g��+2O2��g���T2NO2��g����H=+67.7 kJ/mol
��H2��ԭNO2����ˮ������Ӧ���Ȼ�ѧ����ʽ��
��������1�����ݷ���ʽ�������ɵ����������ʵ�������������������Ũ�ȱ仯������v=
����v��H2����
��2������ͼ��֪�¶���ͬʱ������ƽ��ʱ��ѹǿΪp2��COת���ʸߣ�����ѹǿ��ƽ���Ӱ�������
��������a molCO��2a molH2����ԭ���ij�ʼ�����ȱ������ﵽƽ��ʱ��ԭƽ�����ѹǿ����ƽ�����ƣ�
��3������Ksp��c��CO32-�����c��Ca2+����
��4�������Ȼ�ѧ����ʽ��˹���ɼ���õ���
| ��c |
| ��t |
��2������ͼ��֪�¶���ͬʱ������ƽ��ʱ��ѹǿΪp2��COת���ʸߣ�����ѹǿ��ƽ���Ӱ�������
��������a molCO��2a molH2����ԭ���ij�ʼ�����ȱ������ﵽƽ��ʱ��ԭƽ�����ѹǿ����ƽ�����ƣ�
��3������Ksp��c��CO32-�����c��Ca2+����
��4�������Ȼ�ѧ����ʽ��˹���ɼ���õ���
����⣺��1��10minĩ��0.1mol CO���ɣ����ݷ���ʽCH4��g��+H2O��g��?CO��g��+3H2��g����֪�����ɵ�����Ϊ0.1mol��3=0.3mol�����������Ϊ10L��
��1min����������ʾ��ƽ������v��H2��=
=3.0��10-3mol?L-1?min-1��
�ʴ�Ϊ��3.0��10-3mol?L-1?min-1��
��2������ͼ��֪�¶���ͬʱ������ƽ��ʱ��ѹǿΪp2��COת���ʸߣ�ƽ��������Ӧ�����ƶ�����ӦΪ���������С�ķ�Ӧ������ѹǿƽ���������С�ķ����ƶ�����p1��p2���ʴ�Ϊ������
���������������������£�������a molCO��2a molH2����ԭ���ij�ʼ�����ȱ������ﵽ��ƽ��ʱ��ԭƽ�����ѹǿ����ƽ�����ƣ�����CO��ת�������ʴ�Ϊ������
��3����֪��Һ��c��CO32-��=0.010mol?L-1��Ksp��CaCO3��=c��Ca2+��?c��CO32-��=2.8��10-9������c��Ca2+��=2.8��10-7 mol?L-1��
�𣺸����ӵ�Ũ��Ϊ��2.8��10-7 mol?L-1��
��4����֪����2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol
��N2��g��+2O2��g��=2NO2��g����H=+67.7kJ/mol
�ɸ�˹���ɿ�֪�����2-���4H2��g��+2NO2��g��=N2��g��+4H2O��g����
�ʡ�H=2����-483.6kJ/mol��-67.7kJ/mol=-1034.9kJ/mol�����Ȼ�ѧ����ʽΪ��4H2��g��+2NO2��g��=N2��g��+4H2O��g����H=-1034.9kJ/mol��
�ʴ�Ϊ��4H2��g��+2NO2��g��=N2��g��+4H2O��g����H=-1034.9kJ/mol��
��1min����������ʾ��ƽ������v��H2��=
| ||
| 1min |
�ʴ�Ϊ��3.0��10-3mol?L-1?min-1��
��2������ͼ��֪�¶���ͬʱ������ƽ��ʱ��ѹǿΪp2��COת���ʸߣ�ƽ��������Ӧ�����ƶ�����ӦΪ���������С�ķ�Ӧ������ѹǿƽ���������С�ķ����ƶ�����p1��p2���ʴ�Ϊ������
���������������������£�������a molCO��2a molH2����ԭ���ij�ʼ�����ȱ������ﵽ��ƽ��ʱ��ԭƽ�����ѹǿ����ƽ�����ƣ�����CO��ת�������ʴ�Ϊ������
��3����֪��Һ��c��CO32-��=0.010mol?L-1��Ksp��CaCO3��=c��Ca2+��?c��CO32-��=2.8��10-9������c��Ca2+��=2.8��10-7 mol?L-1��
�𣺸����ӵ�Ũ��Ϊ��2.8��10-7 mol?L-1��
��4����֪����2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol
��N2��g��+2O2��g��=2NO2��g����H=+67.7kJ/mol
�ɸ�˹���ɿ�֪�����2-���4H2��g��+2NO2��g��=N2��g��+4H2O��g����
�ʡ�H=2����-483.6kJ/mol��-67.7kJ/mol=-1034.9kJ/mol�����Ȼ�ѧ����ʽΪ��4H2��g��+2NO2��g��=N2��g��+4H2O��g����H=-1034.9kJ/mol��
�ʴ�Ϊ��4H2��g��+2NO2��g��=N2��g��+4H2O��g����H=-1034.9kJ/mol��
���������⿼���˻�ѧ��Ӧ���ʼ��㡢ƽ���ƶ����Ȼ�ѧ����ʽ����д����˹���ɵ�Ӧ�õȣ��Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ
 KIO3 +3H2����
KIO3 +3H2���� KIO3 +3H2����
KIO3 +3H2����