��Ŀ����
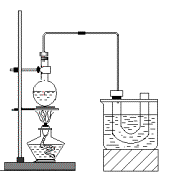
ʵ�����Ʊ������飨C2H5Br����װ�úͲ�������ͼ������֪������ķе�38��4�棩
�ټ��װ�õ������ԣ���װ��ͼ��ʾ��U�ܺʹ��ձ��м����ˮ��
����Բ����ƿ�м���10mL95%�Ҵ���28mLŨ���ᣬȻ�������ϸ��13g�廯�ƺͼ������Ƭ��
��С�ļ��ȣ�ʹ���ַ�Ӧ��
�ش��������⣺
��1����ʵ����ȡ������Ļ�ѧ����ʽΪ�����ɵ���ΪNaHSO4����___________________________��
��2����Ӧʱ���¶ȹ��ߣ��ɿ����к���ɫ�����������ɷ�Ϊ__________��д����ʽ����
��3��Ϊ�˸��õĿ��Ʒ�Ӧ�¶ȣ�����ͼʾ��С����ȣ����õļ��ȷ�ʽ��__________��
��4��U���ڿɹ۲쵽��������_____________________________��
��5����Ӧ������U�ι��д��Ƶ�C2H5Br���ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е����ʣ���ѡ�������Լ��е�_________________������ţ�
�������Ҫ����������______________�����������ƣ���
��6�����м���ʵ�鲽�裬�����ڼ�������������Ԫ�أ�����ȷ�IJ���˳���ǣ�ȡ���������飬Ȼ��__________________������ţ���
�ټ��ȣ��ڼ���AgNO3��Һ���ۼ���ϡHNO3�ữ���ܼ���NaOH��Һ������ȴ
�ټ��װ�õ������ԣ���װ��ͼ��ʾ��U�ܺʹ��ձ��м����ˮ��
����Բ����ƿ�м���10mL95%�Ҵ���28mLŨ���ᣬȻ�������ϸ��13g�廯�ƺͼ������Ƭ��
��С�ļ��ȣ�ʹ���ַ�Ӧ��
�ش��������⣺

��1����ʵ����ȡ������Ļ�ѧ����ʽΪ�����ɵ���ΪNaHSO4����___________________________��
��2����Ӧʱ���¶ȹ��ߣ��ɿ����к���ɫ�����������ɷ�Ϊ__________��д����ʽ����
��3��Ϊ�˸��õĿ��Ʒ�Ӧ�¶ȣ�����ͼʾ��С����ȣ����õļ��ȷ�ʽ��__________��
��4��U���ڿɹ۲쵽��������_____________________________��
��5����Ӧ������U�ι��д��Ƶ�C2H5Br���ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е����ʣ���ѡ�������Լ��е�_________________������ţ�
| A���� | B��H2O | C��Na2SO3��Һ | D��CCl4 |
��6�����м���ʵ�鲽�裬�����ڼ�������������Ԫ�أ�����ȷ�IJ���˳���ǣ�ȡ���������飬Ȼ��__________________������ţ���
�ټ��ȣ��ڼ���AgNO3��Һ���ۼ���ϡHNO3�ữ���ܼ���NaOH��Һ������ȴ
��14�֣���2�֣���1��C2H5OH+NaBr+H2SO4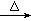 NaHSO4+C2H5Br+H2O
NaHSO4+C2H5Br+H2O
��2��Br2 SO2��3��ˮԡ���ȣ�4������״Һ�����ɣ�5��c ��Һ©�� ��6���ܢ٢ݢۢ�
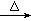 NaHSO4+C2H5Br+H2O
NaHSO4+C2H5Br+H2O ��2��Br2 SO2��3��ˮԡ���ȣ�4������״Һ�����ɣ�5��c ��Һ©�� ��6���ܢ٢ݢۢ�
�����������1��Ũ������廯�Ʒ�Ӧ�����廯�⣬�廯����Ҵ�����ȡ����Ӧ���������飬���ʵ����ȡ������Ļ�ѧ����ʽΪC2H5OH+NaBr+H2SO4
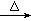 NaHSO4+C2H5Br+H2O��
NaHSO4+C2H5Br+H2O����2������Ũ�������ǿ�����ԣ��������Ӿ��л�ԭ�ԣ���������¶ȹ��ߣ���Ũ�����п��ܰ��������������ɵ����壬��Ũ����Ļ�ԭ������SO2�������������Ǻ���ɫ�ġ�
��3������������ķе�38��4�棬����Ϊ�˸��õĿ��Ʒ�Ӧ�¶ȣ�����ͼʾ��С����ȣ����õļ��ȷ�ʽ��ˮԡ���ȡ�
��4��������ķе�ͣ������ڱ�ˮ����ȴ������̬���Һ̬����U���ڿɹ۲쵽������������״Һ�����ɡ�
��5�����ɵ��������������ܽ��˵������ʹC2H5Br���ػ�ɫ���嵥�ʾ���ǿ�����ԣ�����Ϊ�˳�ȥ�ֲ�Ʒ�е����ʣ�Ӧ��ѡ����л�ԭ�Ե�����������Һ�������鲻����ˮ��ֱ�ӷ�Һ���ɣ��������Ҫ���������Ƿ�Һ©����
��6��Ҫ��������������Ԫ�أ�������Ҫʹ���������������Ƶ�ˮ��Һ��ˮ�⣬Ȼ���ڼ�����������Һ������Ҫע������ڼ�����������Һ֮ǰ������Ҫ���������к��������ƣ�������ȷ�IJ���˳���Ǣܢ٢ݢۢڡ�
������������������Ʊ�Ϊ���壬�ص㿼��ѧ���������Ʊ����˽���������������ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԡ������ۺ���ǿ�������߿������ض�ѧ��������������ѵ��������������ѧ���������������淶�Ͻ���ʵ����������Լ����ֲ�������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ���������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
�����Ŀ