��Ŀ����
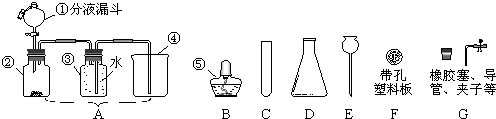
������ͼ�ش����⣺(1)װ���У��ڷ�Ӧǰ�����ƽ�����ƿ��ڼ��װ�õ������ԣ���۲첻�����Ե�����������ʲô�ķ���֤����װ�ò�©��________________________________________________________��
(2)д��Ũ�����ľ̿���ڼ��������·�����Ӧ�Ļ�ѧ����ʽ��________________________��
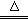
(3)�����ͼ�е�װ�ü���������Ӧ��ȫ�����д������������ʾ��������Ӧ������Լ������Ƽ������ã�
A�м�����Լ���_________��������____________________________________��
B�м�����Լ���_________��������____________________________________��
C�м�����Լ���_________�������dz���__________________���塣
D�м�����Լ���_________��������____________________________________��
(4)ʵ��ʱ��C��Ӧ�۲쵽��������____________________________________��
������(1)��ʵ��װ�ý��������Լ������ѧ��ѧʵ��Ļ���Ҫ��֮һ����ѧѧ���ķ����ǣ���������ƿ�ĵ��ܵ�һ�˽���ˮ�У������ƽ�����ƿ��ڣ�ƿ�ڿ����������ͣ����װ�ò�©�������ܿڻ�������ð�������ƿ�����ƿ��ȴˮ�ͻ������������γ�һ��ˮ���������У�װ�ñȽϴ�˫�ֵ�����������ʹ����װ�����������ͣ������B��C��Dϴ��ƿ�ĵ��ܿڼ���������ð������ϵ����װ�������Ե�Ӧ��ԭ�������ڷ�Ӧǰ��С����ȣ��������ݴ�ϴ��ƿ�ĵ�����ð����ֹͣ���Ⱥ���ƿ��ȴ��ƿ��ѹ����С��ˮ������ϴ��ƿ�ĵ�����γ�һ��ˮ�����ɴ�֤��װ�ò�©����
�𰸣�(1)��Ӧǰ����С�������ƿ��B��C��Dƿ��������ð����ֹͣ���Ⱥ�ˮ�������������γ�һ��ˮ����֤��װ�ò�©��
(2)2H2SO4(Ũ)+C![]() 2SO2��+CO2��+2H2O
2SO2��+CO2��+2H2O
(3)A����ˮ����ͭ������H2O
B��Ʒ����Һ������SO2
C������KMnO4��Һ��SO2
D������ʯ��ˮ������CO2
(4)���Ը��������Һ����ɫ���ʾ�(����ɫ�����ʳ���ɫ)

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�