��Ŀ����
������ͼ�ش����⣺

��1��д��Ũ�����ľ̿���ڼ��������·�����Ӧ�Ļ�ѧ����ʽ��
��2��ʢ��ľ̿������������
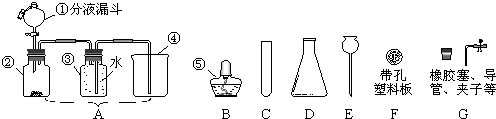
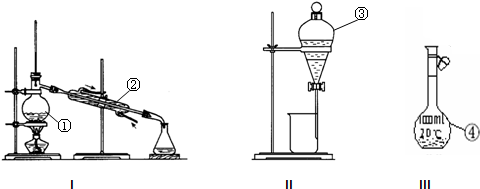
��3�������ͼ�е�װ�ü���������Ӧ��ȫ�����д�������ŵ�������Ӧ������Լ����Ƽ�����;��
A�м�����Լ���
B�м�����Լ���

��1��д��Ũ�����ľ̿���ڼ��������·�����Ӧ�Ļ�ѧ����ʽ��
C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O
| ||
C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O
��
| ||
��2��ʢ��ľ̿������������
Բ����ƿ
Բ����ƿ
ʢװŨ����������������Һ©��
��Һ©��
����B���������ƿ
���ƿ
��3�������ͼ�е�װ�ü���������Ӧ��ȫ�����д�������ŵ�������Ӧ������Լ����Ƽ�����;��
A�м�����Լ���
��ˮ����ͭ��ĩ
��ˮ����ͭ��ĩ
���������������ˮ
�������ˮ
��B�м�����Լ���
Ʒ����Һ
Ʒ����Һ
����������������������
��������������
����������1��Ũ�����ľ̿���ڼ��������·�����Ӧ���ɶ�����̼�����������ˮ��
��2������װ��ͼ����������;�����ش��������ƣ�
��3����֤���ɲ���Ӧ����֤ˮ�����Ĵ��ڣ�����֤����������ڣ���ȥ����������֤���������Ƿ��������֤������̼�Ĵ��ڣ�
��2������װ��ͼ����������;�����ش��������ƣ�
��3����֤���ɲ���Ӧ����֤ˮ�����Ĵ��ڣ�����֤����������ڣ���ȥ����������֤���������Ƿ��������֤������̼�Ĵ��ڣ�
����⣺��1��Ũ�����ľ̿���ڼ��������·�����Ӧ���ɶ�����̼�����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O��
�ʴ�Ϊ��C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O��
��2��װ��ͼ������֪ʢ��ľ̿������������ΪԲ����ƿ�� ʢװŨ���������������Ƿ�Һ©��������B������Ϊ���ƿ��
�ʴ�Ϊ��Բ����ƿ����Һ©�������ƿ��
��3����֤���ɲ���Ӧ������װ��A�е���ˮ����ͭ��֤ˮ�����Ĵ��ڣ�����ˮ����֤������ˮ��������Ʒ����Һ��֤����������ڣ�ͨ��װ��B�е�Ʒ����Һ��ɫ֤�����ɶ�����������ɣ�
�ʴ�Ϊ����ˮ����ͭ��ĩ���������ˮ��Ʒ����Һ����������������
| ||
�ʴ�Ϊ��C+2H2SO4��Ũ��
| ||
��2��װ��ͼ������֪ʢ��ľ̿������������ΪԲ����ƿ�� ʢװŨ���������������Ƿ�Һ©��������B������Ϊ���ƿ��
�ʴ�Ϊ��Բ����ƿ����Һ©�������ƿ��
��3����֤���ɲ���Ӧ������װ��A�е���ˮ����ͭ��֤ˮ�����Ĵ��ڣ�����ˮ����֤������ˮ��������Ʒ����Һ��֤����������ڣ�ͨ��װ��B�е�Ʒ����Һ��ɫ֤�����ɶ�����������ɣ�
�ʴ�Ϊ����ˮ����ͭ��ĩ���������ˮ��Ʒ����Һ����������������
���������⿼����Ũ��������ʵ�ʵ����ƺ����ʷ����жϣ������ʵ����֤�������Լ�ѡ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�
�����Ŀ