��Ŀ����
| |||||||||||||||||||
������
(1) |
|
(2) |
��̪,��Һ��ɫ����ɫͻ�䵽�ۺ�ɫ(������ڲ��ٱ�ɫ) |
(3) |
1,A��B��H |
(4) |
0.118mol/L |

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| |||||||||||||||||||
��12�֣�ʵ���ҳ���ȷ�������ڱ���������أ��ṹ��ʽ��ͼ��ʾ����ȷ�ⶨNaOH����Һ��Ũ�ȣ���������ѧʵ���г���Ϊ���궨����һ�ַ�����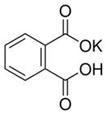
��֪����NaOH��Һ��Ũ����0.1 mol��L��1���ң��ζ��յ�ʱ��Һ��pHӦΪ9.1��
���ڱ������������Է�������Ϊ204
��1��д���ڱ������������NaOH��Ӧ�����ӷ���ʽ________________________��
��2������������ƽ�ƺõ��ڱ���������ط�����ƿ�У���������ˮ�ܽ⣬��Һ����ɫ��
�ټ���ָʾ��______���Ӽ��ȡ���̪��ѡ����NaOH��Һ�ζ����յ�ʱ��������____________��
ijѧ���������Ĵ�ʵ�飬ʵ���������±���
| ʵ���� | �ڱ���������ص�������g�� | ����NaOH��Һ�������mL�� |
| 1 | 0.4080 | 18.20 |
| 2 | 17.10 | |
| 3 | 16.90 | |
| 4 | 17.00 |
A����ʽ�ζ�����װҺǰδ�ô���NaOH��Һ��ϴ2��3��
B���ζ���ʼǰ��ʽ�ζ��ܼ��첿�������ݣ��ڵζ��յ����ʱδ��������
C��ʢ���ڱ������������Һ����ƿ��������ˮ
D���ﵽ�ζ��յ�ʱ��������Һ��Һ����͵����
E���ζ������У���ƿҡ����̫���ң�������ЩҺ�ηɽ�����
F���ζ����յ�ʱ����Һ��ɫ����ɫ�䵽�˺�ɫ
��4����ͬѧ����õ�NaOH��Һ�����ʵ���Ũ��Ϊ________ mol��L��1�����������λС������
��12�֣�ʵ���ҳ���ȷ�������ڱ���������أ��ṹ��ʽ��ͼ��ʾ����ȷ�ⶨNaOH����Һ��Ũ�ȣ���������ѧʵ���г���Ϊ���궨����һ�ַ�����
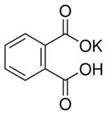
��֪����NaOH��Һ��Ũ����0.1 mol��L��1���ң��ζ��յ�ʱ��Һ��pHӦΪ9.1��
���ڱ������������Է�������Ϊ204
��1��д���ڱ������������NaOH��Ӧ�����ӷ���ʽ________________________��
��2������������ƽ�ƺõ��ڱ���������ط�����ƿ�У���������ˮ�ܽ⣬��Һ����ɫ��
�ټ���ָʾ��______���Ӽ��ȡ���̪��ѡ����NaOH��Һ�ζ����յ�ʱ��������____________��
ijѧ���������Ĵ�ʵ�飬ʵ���������±���
|
ʵ���� |
�ڱ���������ص�������g�� |
����NaOH��Һ�������mL�� |
|
1 |
0.4080 |
18.20 |
|
2 |
17.10 |
|
|
3 |
16.90 |
|
|
4 |
17.00 |
��3���ζ������ϴ���ǵ�________��ʵ�飬����������Ŀ���ԭ����____________��
A����ʽ�ζ�����װҺǰδ�ô���NaOH��Һ��ϴ2��3��
B���ζ���ʼǰ��ʽ�ζ��ܼ��첿�������ݣ��ڵζ��յ����ʱδ��������
C��ʢ���ڱ������������Һ����ƿ��������ˮ
D���ﵽ�ζ��յ�ʱ��������Һ��Һ����͵����
E���ζ������У���ƿҡ����̫���ң�������ЩҺ�ηɽ�����
F���ζ����յ�ʱ����Һ��ɫ����ɫ�䵽�˺�ɫ
��4����ͬѧ����õ�NaOH��Һ�����ʵ���Ũ��Ϊ________ mol��L��1�����������λС������
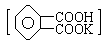 ��ȷ�ⶨNaOH����Һ��Ũ�ȣ���������ѧʵ���г���Ϊ���궨����һ�ַ����������ϲ�ã��ζ��յ��pHӦΪ9.1���Իش�
��ȷ�ⶨNaOH����Һ��Ũ�ȣ���������ѧʵ���г���Ϊ���궨����һ�ַ����������ϲ�ã��ζ��յ��pHӦΪ9.1���Իش�