��Ŀ����
����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�
��1����AΪŨ���ᣬBΪ�������ڽ���Ԫ�ص�Ƭ״���ʣ����ڳ���������ˮ��Ӧ��CΪƷ����Һ��ʵ���й۲쵽��Һ��ɫ����B��______��д��ѧʽ����B��ŨH2SO4��Ӧ�Ļ�ѧ����ʽΪ______����Ӧ�����ձ��м����ˮ���ֿɹ۲쵽�Թ�C�е�����Ϊ______��
��2����BΪNa2CO3��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ����ǣ�����AӦ���е�������______��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е�������______��
��3����B����ʯ�ң�ʵ���й۲쵽C��Һ���γɳ�����Ȼ������ܽ⣬����Һǡ�ó���ʱ���ر�E��Ȼ�����ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��______��д���ƣ���C��______��д��ѧʽ���������ǵĻ��Һ���÷�Ӧ�����ӷ���ʽΪ______������D�ڴ�ʵ���е�������______��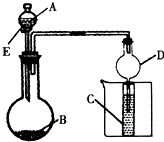
��1����AΪŨ���ᣬBΪ�������ڽ���Ԫ�ص�Ƭ״���ʣ����ڳ���������ˮ��Ӧ��CΪƷ����Һ��ʵ���й۲쵽��Һ��ɫ����B��______��д��ѧʽ����B��ŨH2SO4��Ӧ�Ļ�ѧ����ʽΪ______����Ӧ�����ձ��м����ˮ���ֿɹ۲쵽�Թ�C�е�����Ϊ______��
��2����BΪNa2CO3��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ����ǣ�����AӦ���е�������______��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е�������______��
��3����B����ʯ�ң�ʵ���й۲쵽C��Һ���γɳ�����Ȼ������ܽ⣬����Һǡ�ó���ʱ���ر�E��Ȼ�����ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��______��д���ƣ���C��______��д��ѧʽ���������ǵĻ��Һ���÷�Ӧ�����ӷ���ʽΪ______������D�ڴ�ʵ���е�������______��

��1���������֪��BΪ�������ڽ���Ԫ�ص�Ƭ״���ʣ����ڳ���������ˮ��Ӧ��B����Ϊ������þ����Ũ���᳣���»�ʹ���ۻ�������Bֻ��Ϊþ����Ũ���ᷢ��������ԭ��Ӧ����Ӧ�ķ���ʽΪMg+2H2SO4��Ũ��=MgSO4+SO2��+2H2O��SO2����Ư���ԣ���ʹƷ����ɫ�������ȶ����������ָܻ���ԭ������ɫ��
�ʴ�Ϊ��Mg��Mg+2H2SO4��Ũ��=MgSO4+SO2��+2H2O��C����Һ��죻
��2���۲쵽С�Թ�����Һ����ǣ�˵�����ɶ�����̼����A������Ӧ��̼�������ǿ�����ɱ�����������ˮ��������Һ����壬
�ʴ�Ϊ�����Ա�̼�������ǿ����Һ�ɻ��DZ���壻
��3���Թܱڳ��ֹ�����������˵�����ɰ���������Ũ��ˮ����ε�Ũ��Һ����ʯ�ҷ�Ӧ��ȡ��C��ӦΪ��������������Һ�ķ�Ӧ����C��ӦΪAgNO3�������ǵĻ��Һ������ʱ������CH2OH��CHOH��4CHO+2[Ag��NH3��2]++2OH-
CH2OH��CHOH��4COO-+NH4++H2O+2Ag+3NH3����װ��D����ֹ���������ã�
�ʴ�Ϊ��Ũ��ˮ������ε�Ũ��Һ����AgNO3��CH2OH��CHOH��4CHO+2[Ag��NH3��2]++2OH-
CH2OH��CHOH��4COO-+NH4++H2O+2Ag+3NH3������ֹ������
�ʴ�Ϊ��Mg��Mg+2H2SO4��Ũ��=MgSO4+SO2��+2H2O��C����Һ��죻
��2���۲쵽С�Թ�����Һ����ǣ�˵�����ɶ�����̼����A������Ӧ��̼�������ǿ�����ɱ�����������ˮ��������Һ����壬
�ʴ�Ϊ�����Ա�̼�������ǿ����Һ�ɻ��DZ���壻
��3���Թܱڳ��ֹ�����������˵�����ɰ���������Ũ��ˮ����ε�Ũ��Һ����ʯ�ҷ�Ӧ��ȡ��C��ӦΪ��������������Һ�ķ�Ӧ����C��ӦΪAgNO3�������ǵĻ��Һ������ʱ������CH2OH��CHOH��4CHO+2[Ag��NH3��2]++2OH-
| ˮ�� |
| �� |
�ʴ�Ϊ��Ũ��ˮ������ε�Ũ��Һ����AgNO3��CH2OH��CHOH��4CHO+2[Ag��NH3��2]++2OH-
| ˮ�� |
| �� |

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
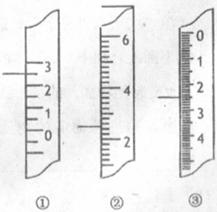 (4��)��(1)�¶ȼƳ�����������ƻ�ѧʵ����¶ȡ�
(4��)��(1)�¶ȼƳ�����������ƻ�ѧʵ����¶ȡ� C�����ǵζ��ܣ�����Ϊ3.5 mL
C�����ǵζ��ܣ�����Ϊ3.5 mL �ֽ�6.0molCO2��8.0molH2����2L���ܱ������У����H2�����ʵ�����n����ʱ��仯����ͼ��ʾ��ʵ�ߣ���
�ֽ�6.0molCO2��8.0molH2����2L���ܱ������У����H2�����ʵ�����n����ʱ��仯����ͼ��ʾ��ʵ�ߣ���
 ���Դ˵������Դ����ʵ������ģ������Ʒ����
���Դ˵������Դ����ʵ������ģ������Ʒ����