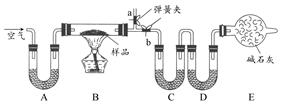
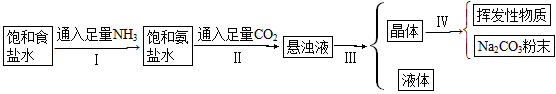
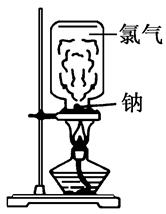
��Ŀ����
ij��������Ƶ���Ա�Ըó�������Ʒ���������м��顣�����ܹ�˵��������Ʒ�п��ܺ�������(NaHCO3)��ʵ����( )
����Ʒ����ˮ�������������ij���ʯ��ˮ�������
����Ʒ����ˮ��������������BaCl2��Һ�������
����Ʒ��Ӳ���Թ��м�ǿ�ȣ��ų�������ͨ�����ʯ��ˮ�������
������Ʒ�еμ�ϡ���ᣬ�ų�������ͨ�����ʯ��ˮ�������
����Ʒ����ˮ�������������ij���ʯ��ˮ�������
����Ʒ����ˮ��������������BaCl2��Һ�������
����Ʒ��Ӳ���Թ��м�ǿ�ȣ��ų�������ͨ�����ʯ��ˮ�������
������Ʒ�еμ�ϡ���ᣬ�ų�������ͨ�����ʯ��ˮ�������
| A���٢ڢ� | B���٢ۢ� | C���٢ڢ� | D��ֻ�Т� |
D
���ݴ���(Na2CO3)��NaHCO3�����ʲ�ͬ��������Na2CO3��NaHCO3������Ca(OH)2��Ӧ������������Na2CO3��Ca(OH)2=CaCO3����2NaOH,2NaHCO3��Ca(OH)2=CaCO3����2H2O��Na2CO3��Na2CO3��NaHCO3���������ᷴӦ�ų����壬��Na2CO3��2HCl=2NaCl��H2O��CO2����NaHCO3��HCl=NaCl��H2O��CO2�������Ԣ١��ܲ���˵����Ʒ���Ƿ���NaHCO3������BaCl2��Na2CO3=BaCO3����2NaCl����NaHCO3������BaCl2��Ӧ�����Ԣ��л�������ֻ��˵��Na2CO3һ�����ڣ�����ȷ���Ƿ���NaHCO3����2NaHCO3 Na2CO3��H2O��CO2����Na2CO3���Ȳ��ֽ⣬���Ԣ�������һ����˵����NaHCO3���ڡ�
Na2CO3��H2O��CO2����Na2CO3���Ȳ��ֽ⣬���Ԣ�������һ����˵����NaHCO3���ڡ�
 Na2CO3��H2O��CO2����Na2CO3���Ȳ��ֽ⣬���Ԣ�������һ����˵����NaHCO3���ڡ�
Na2CO3��H2O��CO2����Na2CO3���Ȳ��ֽ⣬���Ԣ�������һ����˵����NaHCO3���ڡ�
��ϰ��ϵ�д�
 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
�����Ŀ