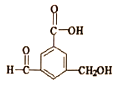
��Ŀ����
����Ŀ�����±�ɫ����������������ܽ���Ϊ�г����³裬��ͼ��ʾ���Ĥ�IJ������±�ɫϵͳ���乤��ԭ��������ӵ�Դ�£�ͨ����Ĥ�����ڲ�����������ԭ��Ӧ��ʵ�ֶ������Ĺ����ʽ��ж༶�����Ե��ڡ�(��֪��WO3�� Li4 Fe4[Fe(CN)6]3��Ϊ��ɫ����LiWO3��Fe4[Fe(CN)6]3��Ϊ��ɫ)�����й�˵����ȷ����
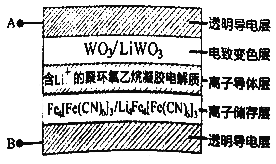
A. ��A��ӵ�Դ����ʱ����ʱLi+�õ����ӱ���ԭ
B. ��A��ӵ�Դ����ʱ��Ĥ�����ʽ��ͣ�������Ч�赲����
C. ��B��ӵ�Դ����ʱ�����ӵ������Li+����Ǩ��
D. ��B��ӵ�Դ����ʱ�����Ӵ���㷢����ӦΪ��Fe4[Fe(CN)6]3+4Li++4e��=Li4Fea[Fe(CN)6]3
���𰸡�D
��������
A����A����ӵ�Դ����ʱ��AΪ������������ԭ��ӦΪWO3+ Li++e-= LiWO3����A������
B����A����ӵ�Դ����ʱ��A�����������ڵ��ص������Ϸ���ʧ���ӵ�������Ӧ�����±�ɫ�㷢����ӦΪ��LiWO3-e-= WO3+ Li+������WO3��������Ϊ��ɫ��������������Ч�赲���⣬��B����
C����B��ӵ�Դ����ʱ��B���������������������������������ӵ������Li+����Ǩ�ƣ���C����
D����B����ӵ�Դ����ʱ��BΪ��������ʱ���Ӵ��������ɫ��Fe4[Fe(CN)6]3�õ��ӳ�ΪLi4Fea[Fe(CN)6]3��������ӦΪ��Fe4[Fe(CN)6]3+4Li++4e��=Li4Fea[Fe(CN)6]3����D��ȷ��
��ѡD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�