��Ŀ����
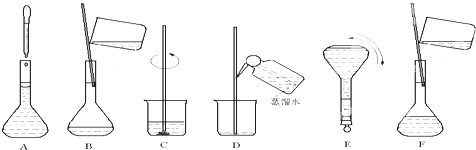
��ͼ��ʾ����100mL 0.100mol?L-1 Na2CO3��Һ�ļ����ؼ�ʵ�鲽��Ͳ�������ͼ�ش��������⣺

��1������Na2CO3?10H2O��������Һ����Ҫ��������
��2��д��������Һ������Ҫ�õ��IJ������������ƣ��ձ���
��3������Bͨ����Ϊת�ƣ�����Aͨ����Ϊ
��4��������ʵ�鲽��A-F��ʵ������Ⱥ��������

��1������Na2CO3?10H2O��������Һ����Ҫ��������
2.9
2.9
�ˣ�����ȡ�ľ����Ѿ���һ����ʧȥ�˽ᾧˮ���������Ƶ���ҺŨ��ƫ��
��
�������/С��������2��д��������Һ������Ҫ�õ��IJ������������ƣ��ձ���
100mL����ƿ
100mL����ƿ
������������ͷ�ι�
����������ͷ�ι�
����3������Bͨ����Ϊת�ƣ�����Aͨ����Ϊ
����
����
��������ӿ̶��ߣ����Ƶ�Ũ�Ƚ�ƫ��
��
������С��������D��Ϊϴ��
ϴ��
����û�в���D�������Ƶ�Ũ��ƫС
С
�������/С��������4��������ʵ�鲽��A-F��ʵ������Ⱥ��������
C��B��D��F��A��E
C��B��D��F��A��E
����������1������n=cv��������Na2CO3�����ʵ���������Na2CO3?10H2O�����ʵ�������Na2CO3�����ʵ���������m=nM����Na2CO3?10H2O������������c=
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��3���ý�ͷ�ιܵμ���Һ�IJ��������Ƕ��ݣ�������ˮϴ�Ӳ������IJ���������ϴ�ӣ�����c=
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��4������������Һ��ʵ��������̽���ʵ�鲽������
| n |
| V |
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��3���ý�ͷ�ιܵμ���Һ�IJ��������Ƕ��ݣ�������ˮϴ�Ӳ������IJ���������ϴ�ӣ�����c=
| n |
| V |
��4������������Һ��ʵ��������̽���ʵ�鲽������
����⣺��1��ʵ��������100mL 0.100mol?L-1 Na2CO3��Һ��ҪNa2CO3�����ʵ���Ϊ��0.1L��0.1mol/L=0.01mol��Na2CO3?10H2O�����ʵ���Ϊ0.01mol��Na2CO3?10H2O������Ϊ��0.01mol��286g/mol=2.86g������ƽ�ľ�ȷ��Ϊ0.1g��������Ҫ��������Ϊ2.9g������ȡ�ľ����Ѿ���һ����ʧȥ�˽ᾧˮ������̼���Ƶ�����ƫ��nƫ����c=
������֪��Һ��Ũ��ƫ��
����2.9����
��2�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��100mL����ƿ����ͷ�ιܡ���������
��3����ˮ��Һ�����̶���1��2cmʱ���ý�ͷ�ι�������ƿ�еμ���Һ���ò����������Ƕ��ݣ�
������ӿ̶��ߣ���Һ�����ƫС������c=
������֪���Ƶ�Ũ�Ƚ�ƫ��
ת�ƺ�������ˮϴ�Ӳ������IJ���������ϴ�ӣ�
��û��ϴ�ӣ����ʵ����ʵ���ƫС������c=
������֪���Ƶ�Ũ�Ƚ�ƫС��
�ʴ�Ϊ�����ݣ���ϴ�ӣ�С��
��4�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ʵ������Ⱥ��������Ϊ��C��B��D��F��A��E��
�ʴ�Ϊ��C��B��D��F��A��E��
| n |
| V |
����2.9����
��2�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��100mL����ƿ����ͷ�ιܡ���������
��3����ˮ��Һ�����̶���1��2cmʱ���ý�ͷ�ι�������ƿ�еμ���Һ���ò����������Ƕ��ݣ�
������ӿ̶��ߣ���Һ�����ƫС������c=
| n |
| V |
ת�ƺ�������ˮϴ�Ӳ������IJ���������ϴ�ӣ�
��û��ϴ�ӣ����ʵ����ʵ���ƫС������c=
| n |
| V |
�ʴ�Ϊ�����ݣ���ϴ�ӣ�С��
��4�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ʵ������Ⱥ��������Ϊ��C��B��D��F��A��E��
�ʴ�Ϊ��C��B��D��F��A��E��
���������⿼��һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע��ʵ�鲽�衢������Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ