��Ŀ����
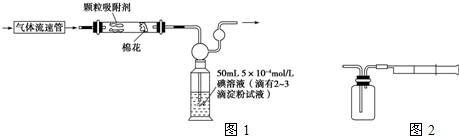
��ͼ��ʾ���� 100mL 0.100mol?L-1 Na2CO3��Һ�ļ����ؼ�ʵ�鲽��Ͳ�������ͼ�ش��������⣺

��1����������ƽ��ȡNa2CO3?10H2O ��������
��2������E�н�һ�����������µߵ����Σ���������������
��3������Bͨ����Ϊת�ƣ�����Aͨ����Ϊ
��4��������ʵ�鲽��A��F��ʵ������Ⱥ��������

��1����������ƽ��ȡNa2CO3?10H2O ��������
2.9g
2.9g
����2������E�н�һ�����������µߵ����Σ���������������
����ƿ
����ƿ
����3������Bͨ����Ϊת�ƣ�����Aͨ����Ϊ
����
����
����4��������ʵ�鲽��A��F��ʵ������Ⱥ��������
DCBFAE
DCBFAE
����������1������m=nM=cvM���㣻
��2������һ�����ʵ���Ũ����Һ������������ƿ��
��3���ý�ͷ�ιܵμ���Һ�IJ��������Ƕ��ݣ�
��4������������Һ��ʵ��������̽���ʵ�鲽������
��2������һ�����ʵ���Ũ����Һ������������ƿ��
��3���ý�ͷ�ιܵμ���Һ�IJ��������Ƕ��ݣ�
��4������������Һ��ʵ��������̽���ʵ�鲽������
����⣺��1��ʵ��������100mL 0.100mol?L-1 Na2CO3��Һ��ҪNa2CO3?10H2O������Ϊ��0.1L��0.1mol/L��286g/mol=2.9g���ʴ�Ϊ��2.9g��
��2������һ�����ʵ���Ũ����Һ������������ƿ�����Ը�����������������ƿ���ʴ�Ϊ������ƿ��
��3����ˮ��Һ�����̶���1��2cmʱ���ý�ͷ�ι�������ƿ�еμ���Һ���ò����������Ƕ��ݣ��ʴ�Ϊ�����ݣ�
��4�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ʵ������Ⱥ��������Ϊ��DCBFAE���ʴ�Ϊ��DCBFAE��
��2������һ�����ʵ���Ũ����Һ������������ƿ�����Ը�����������������ƿ���ʴ�Ϊ������ƿ��
��3����ˮ��Һ�����̶���1��2cmʱ���ý�ͷ�ι�������ƿ�еμ���Һ���ò����������Ƕ��ݣ��ʴ�Ϊ�����ݣ�
��4�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ʵ������Ⱥ��������Ϊ��DCBFAE���ʴ�Ϊ��DCBFAE��
���������⿼��һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע��ʵ�鲽�衢������������ƿ��ѡȡ��Ϊ�״��㣮

��ϰ��ϵ�д�
 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
�����Ŀ
 ���ᣨH2C2O4����һ����Ҫ�Ļ�����Ʒ�����ᾧ�����ɿ���H2C2O4?xH2O��ʾ��Ϊ�˲ⶨxֵ��������ʵ�飺
���ᣨH2C2O4����һ����Ҫ�Ļ�����Ʒ�����ᾧ�����ɿ���H2C2O4?xH2O��ʾ��Ϊ�˲ⶨxֵ��������ʵ�飺