��Ŀ����
����Ŀ��������ͼת���ش��й����⣺
��1��д�����з�Ӧ�Ļ�ѧ����ʽ��
��A��C��Ӧ������������________________
��Aת��ΪB__________________________
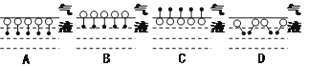
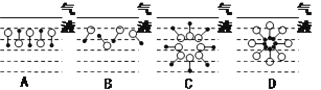
��2��Ϊ����ȡ�����������ס��ҡ�����λͬѧ�ֱ������������ʵ��װ�ã�

��Ӽס�����λͬѧ��Ƶ�װ����ѡ��һ����Ϊʵ������ȡ����������װ�ã�ѡ���װ����________(ѡ��ס����ҡ�)����ͬѧ����װ���еIJ����ܸij����θ���ܣ��������������⣬��һ��Ҫ������___________________��
��3���Թ�B�з�����DZ���____________��Һ�����Թ�B�з���������������õ���Ҫ������__________________________��
���𰸡� CH3COOH+C2H5OH![]() CH3COOC2H5+H2O 2CH3CH2OH+O2
CH3COOC2H5+H2O 2CH3CH2OH+O2![]() 2CH3CHO+2H2O �� ������ ̼���� ��Һ©��
2CH3CHO+2H2O �� ������ ̼���� ��Һ©��
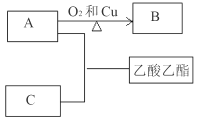
����������1������ͼ����Ϣ��֪AΪ�Ҵ���CΪ���ᣬBΪ��ȩ�����A��C��Ӧ�������������ķ�Ӧ�ķ���ʽΪCH3COOH+C2H5OH![]() CH3COOC2H5+H2O����Aת��ΪB�ķ�Ӧ�ķ���ʽΪ2CH3CH2OH+O2
CH3COOC2H5+H2O����Aת��ΪB�ķ�Ӧ�ķ���ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O����2��������Ҵ�������ˮ��������Һ������Ϊ�˷�ֹ����������ѡ��װ�ã����θ���ܵ�����ͬʱҲ���������ã���3���Թ�B�з�����DZ���̼������Һ���������Ҵ����к����ᡢ���������������ܽ⣻���Թ�B�з���������������õ���Ҫ�����Ƿ�Һ©����
2CH3CHO+2H2O����2��������Ҵ�������ˮ��������Һ������Ϊ�˷�ֹ����������ѡ��װ�ã����θ���ܵ�����ͬʱҲ���������ã���3���Թ�B�з�����DZ���̼������Һ���������Ҵ����к����ᡢ���������������ܽ⣻���Թ�B�з���������������õ���Ҫ�����Ƿ�Һ©����

 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д�����Ŀ��ú������Һ�����ִ���Դ��ҵ���ص㿼�ǵ���Դ�ۺ����÷������������������Ϊ��ú����ˮú��������ǰ�Ƚ����е�Һ������Ϊ��ú����CH 3OH����֪�Ʊ��״����йػ�ѧ��Ӧ��ƽ�ⳣ�����£���CO 2(g)��3H 2(g)=CH 3OH(g)��H 2 O(g) �� H 1 =-90.8KJ/mol����CO(g)��H2O(g)=CO2(g)��H2(g) �� H 2 =-41.2kJ/mol,��CO(g)��2H2(g)=CH3OH(g)�� H 3 850 ��ƽ�ⳣ���ֱ�Ϊk1=160�� K2��243��K3=160���״����������ᷴӦ������CH 3OH��l��+CH3COOH��l��![]() CH3COOCH3��l��+H2O��l��
CH3COOCH3��l��+H2O��l��
��1����Ӧ�� H 3�� ___________ �����ϵ�K�ı���ʽ_____________
��2����CO�ϳɼ״�ʱ�������йظ÷�Ӧ��˵����ȷ����________(�����)��
A�����¡����������£��������ڵ�ѹǿ�������仯������淴Ӧ�ﵽƽ��
B��һ�������£�H 2 ������������CO���������ʵ�2��ʱ�����淴Ӧ�ﵽƽ��
C��ʹ�ú��ʵĴ��������̴ﵽƽ���ʱ�䲢���CH 3OH�IJ���
D��ij�¶��£���2 mol CO��6 mol H 2 ����2 L�ܱ������У���ַ�Ӧ���ﵽƽ����c (CO)��0.2 mol��L �� 1 ����CO��ת����Ϊ80%
��3��850 ��ʱ�����ܱ������н��з�Ӧ�ٿ�ʼʱֻ����CO2 ��H 2����Ӧ10min���ø���ֵ�Ũ�����±Ƚ����淴Ӧ�����ʵĴ�С��
v�� __________v��(���������)��ʱ����ڷ�Ӧ����v(H2)�� __________
���� | H 2 | CO 2 | CH 3 OH | H 2 O |
Ũ��(mol/L) | 0.2 | 0.2 | 0.4 | 0.4 |
��4����һ��������3L�����ܱ�������,����һ������H2��CO2��������Ӧ�٣�ʵ���÷�Ӧ���ڲ�ͬ��ʼͶ�����£���Ӧ��ϵ��CO2��ƽ��ת�������¶ȵĹ�ϵ���ߣ���ͼ1��ʾ��
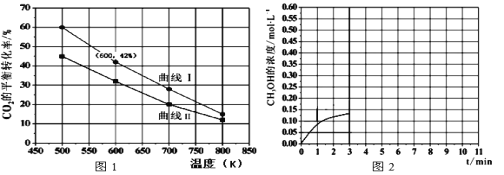
��H2��CO2����ʼ��Ͷ������A��B���ַ�ʽͶ��
A��n(H2)=3mol��n(CO2)=1.5mol
B��n(H2)=3mol��n(CO2)=2mol������I��������Ͷ�뷽ʽ____����A��B��ʾ��
�����¶�Ϊ500K�������£�����A��ʽ����3mol H2��1.5mol CO2���÷�Ӧ10minʱ�ﵽƽ�⣬�ڴ������£�ϵͳ��CH3OH��Ũ���淴Ӧʱ��ı仯������ͼ2��ʾ������Ӧʱ��ﵽ3minʱ��Ѹ�ٽ���ϵ�¶�����600K������ͼ2�л���3��10min��������CH3OHŨ�ȵı仯��������___��