��Ŀ����
��15�֣�
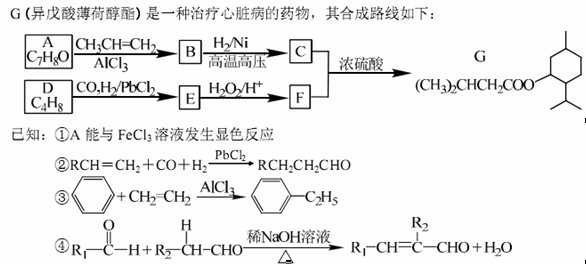
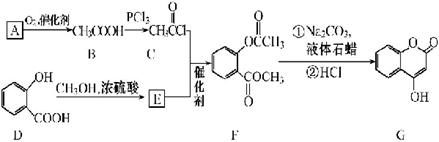
��1�� A������Ϊ�ߣߣߣ�
��2�� G�к�������������Ϊ�ߣߣߣ�
��3�� D�ķ����к��Уߣߣߣ��ֲ�ͬ��ѧ��������ԭ��
��4�� E�����Ƶ�������ͭ��Ӧ�Ļ�ѧ����ʽΪ�ߣߣߣ�
��5��д����������������A������ͬ���칹��Ľṹ��ʽ���ߣߣߣ�
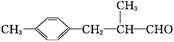
A����������6��̼ԭ����һ��ֱ���ϣ�b�������к�����OH
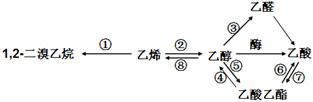
��6�����촼���������ϡ��ٽ����ȣ�д�����Ҵ�Ϊԭ���Ʊ�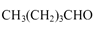 �ĺϳ�·����
�ĺϳ�·����
��ͼ�����Լ����ã����ϳ�·������ʾ��ͼʾ�����£�
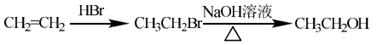

��1�� A������Ϊ�ߣߣߣ�
��2�� G�к�������������Ϊ�ߣߣߣ�
��3�� D�ķ����к��Уߣߣߣ��ֲ�ͬ��ѧ��������ԭ��
��4�� E�����Ƶ�������ͭ��Ӧ�Ļ�ѧ����ʽΪ�ߣߣߣ�
��5��д����������������A������ͬ���칹��Ľṹ��ʽ���ߣߣߣ�
A����������6��̼ԭ����һ��ֱ���ϣ�b�������к�����OH
��6�����촼���������ϡ��ٽ����ȣ�д�����Ҵ�Ϊԭ���Ʊ�
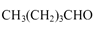 �ĺϳ�·����
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ʾ��ͼʾ�����£�
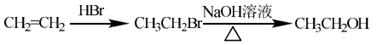
��15�֣�
��1��3�������ӣ��������ӣ���2�֣� ��2��������1�֣�
��3��2��2�֣�
��4��(CH3)2CHCH2CHO+2Cu(OH)2+NaOH (CH3)2CHCH2COONa+Cu2O��+3H2O
(CH3)2CHCH2COONa+Cu2O��+3H2O

��1��3�������ӣ��������ӣ���2�֣� ��2��������1�֣�
��3��2��2�֣�
��4��(CH3)2CHCH2CHO+2Cu(OH)2+NaOH
 (CH3)2CHCH2COONa+Cu2O��+3H2O
(CH3)2CHCH2COONa+Cu2O��+3H2O 
���������A����FeCl3��Һ������ɫ��Ӧ��˵��A���з��ǻ����Ա�G�Ľṹ���ɵ�A�Ľṹ��ʽΪ��
 ������Ϣ�ۣ�A��CH3CH=CH2��AlCl3�����·�����������Ӧ���õ�BΪ��
������Ϣ�ۣ�A��CH3CH=CH2��AlCl3�����·�����������Ӧ���õ�BΪ�� ������Ϣ�ں�G�Ľṹ��֪DΪ��(CH3)2C=CH2��D��CO��H2��PbCl2���������ɵ�EΪ��(CH3)2CHCH2CHO��E��H2O2����Ϊ���ᣬFΪ��(CH3)2CH CH2COOH��
������Ϣ�ں�G�Ľṹ��֪DΪ��(CH3)2C=CH2��D��CO��H2��PbCl2���������ɵ�EΪ��(CH3)2CHCH2CHO��E��H2O2����Ϊ���ᣬFΪ��(CH3)2CH CH2COOH����1��A�Ľṹ��ʽΪ��
 ������Ϊ��3�������ӣ��������ӣ���
������Ϊ��3�������ӣ��������ӣ�����2������G�Ľṹ��ʽ��֪G�к�������������Ϊ������
��3��D�Ľṹ��ʽΪ��(CH3)2C=CH2������D�ķ����к���2�ֲ�ͬ��ѧ��������ԭ�ӡ�
��4��EΪ��(CH3)2CHCH2CHO������ȩ�����������Ƶ�������ͭ��������ѧ����ʽΪ��
(CH3)2CHCH2CHO+2Cu(OH)2+NaOH
 (CH3)2CHCH2COONa+Cu2O��+3H2O
(CH3)2CHCH2COONa+Cu2O��+3H2O��5������a����������6��̼ԭ����һ��ֱ���ϣ�b�������к�����OH����֪a���Ӻ�������̼̼���������������ֽṹ����CH3��C��C��C��C��CH2��CH2OH����CH3��C��C��C��C��C(OH)��CH3����CH3��CH2��C��C��C��C��CH2OH
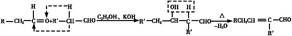
��6��CH3CH2OH�ڴ�����������O2��Ӧ����CH3CHO��������Ϣ�ܣ�CH3CHO������ȩ����ת��Ϊ��CH3CH=CHCHO����H2�ӳ�ת��ΪCH3CH2CH2CH2OH��������ȥ��Ӧ����CH3CH2CH=CH2��������Ϣ�ڣ���CO��H2��PbCl2����������CH3CH2CH2CH2CHO�����Ժϳ�·������ʾ��ͼΪ��


��ϰ��ϵ�д�
�����Ŀ

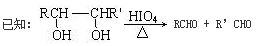
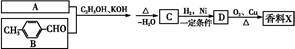
 ����һ��ҽҩ�ϳ��м��壬ijͬѧ������ĺϳ�·�����£�
����һ��ҽҩ�ϳ��м��壬ijͬѧ������ĺϳ�·�����£�
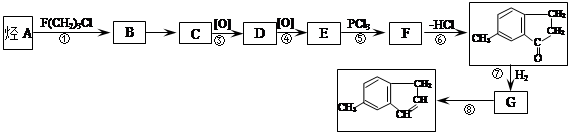

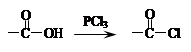

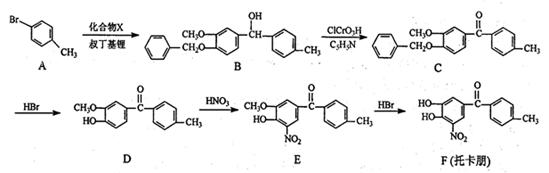
 ���������Ϣ��д���ԶԼ����Ӻ������������
���������Ϣ��д���ԶԼ����Ӻ������������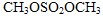 ��Ϊԭ�ϣ��Ʊ�
��Ϊԭ�ϣ��Ʊ� �ĺϳ�·������ͼ(���Լ�����)��
�ĺϳ�·������ͼ(���Լ�����)��
 �ĸ߾���䵥��Ӧ�ǣ��ٱ���ϩ���ڱ�ϩ����2��ϩ����1��ϩ������ϩ���ޱ���ϩ( )
�ĸ߾���䵥��Ӧ�ǣ��ٱ���ϩ���ڱ�ϩ����2��ϩ����1��ϩ������ϩ���ޱ���ϩ( )