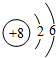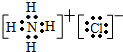��Ŀ����
��1���ڢ�A����A��Ԫ����ɵĻ�����GaN��GaP��GaAs�����˹��ϳɵ����Ͱ뵼����ϣ��侧��ṹ�뵥�������ƣ�Gaԭ�ӵĵ����Ų�ʽΪ ����GaN�����У�ÿ��Gaԭ���� ��Nԭ����������ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ �����Ĵ��������У�GaN���� ���壮NԪ��ԭ�ӵļ۲���ӵĹ����ʾʽΪ ��
��2���˹�ģ��ø�ǵ�ǰ�о����ȵ㣮���о�������������X�������о�ģ��ø�������� ��C
��C
��������ͼ�����ʾ���ϼ�Ϊ+1��ʱ���ֱ��γ�a��b��
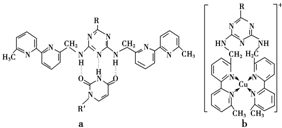
��a���������ں�N�ӻ���̼̼��������ת��˵����̼̼������ �������ԣ�
��C��N��H��O����Ԫ�صĵ縺����С�����˳��Ϊ ��C��O���ɵij�����CO2�ĵ���ʽΪ ��д��N��O�γɺ�CO2�ĵȵ��������ʵĻ�ѧʽΪ ��
�����������ð�����ѧ���ͷ��Ӽ�����ã��Ƚ�a��b��������������IJ��� ��
����a�����������Ϊ���Ӽ�����ͷ��»�����b������λ����
��2���˹�ģ��ø�ǵ�ǰ�о����ȵ㣮���о�������������X�������о�ģ��ø��������
 ��C
��C��������ͼ�����ʾ���ϼ�Ϊ+1��ʱ���ֱ��γ�a��b��

��a���������ں�N�ӻ���̼̼��������ת��˵����̼̼������
��C��N��H��O����Ԫ�صĵ縺����С�����˳��Ϊ
�����������ð�����ѧ���ͷ��Ӽ�����ã��Ƚ�a��b��������������IJ���
����a�����������Ϊ���Ӽ�����ͷ��»�����b������λ����
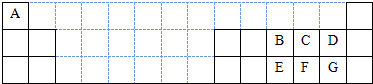
��������1��Ga��31��Ԫ�أ����̬ԭ�Ӻ�����31�����ӣ����ݹ���ԭ����д��ԭ�Ӻ�������Ų�ʽ��
��������Si����������ռ������������״�ṹ��Ϊԭ�Ӿ��壬GaN����ṹ�뵥�������ƣ�GaN����ԭ�Ӿ��壬��ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ�뵫������Si�Ľṹ���ƣ�
����GaN�Ĺ�����ȷ���������ͣ�
��ԭ�Ӻ�����7�����ӣ��������5�����ӣ��������Ӿ���NԪ�ؼ۵��ӣ�
��2���٦Ҽ�������ת���м�������ת��
��Ԫ�صķǽ�����Խǿ����縺��Խǿ��ͬһ�����У�Ԫ�صĵ縺������ԭ���������������������̼������ÿ����ԭ�Ӻ�̼ԭ���γɹ���˫����
ԭ�Ӹ�����ȼ۵�������ȵ���Ϊ�ȵ����壻
��a�к��к��������b�к�����λ����
��������Si����������ռ������������״�ṹ��Ϊԭ�Ӿ��壬GaN����ṹ�뵥�������ƣ�GaN����ԭ�Ӿ��壬��ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ�뵫������Si�Ľṹ���ƣ�
����GaN�Ĺ�����ȷ���������ͣ�
��ԭ�Ӻ�����7�����ӣ��������5�����ӣ��������Ӿ���NԪ�ؼ۵��ӣ�
��2���٦Ҽ�������ת���м�������ת��
��Ԫ�صķǽ�����Խǿ����縺��Խǿ��ͬһ�����У�Ԫ�صĵ縺������ԭ���������������������̼������ÿ����ԭ�Ӻ�̼ԭ���γɹ���˫����
ԭ�Ӹ�����ȼ۵�������ȵ���Ϊ�ȵ����壻
��a�к��к��������b�к�����λ����
����⣺��1��Gaԭ����31��Ԫ�أ�Gaԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s24p1��GaN����ṹ�뵥�������ƣ�GaN����ԭ�Ӿ��壬ÿ��Gaԭ����4��Nԭ����������ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ�������壬��ԭ�Ӻ�����7�����ӣ��������5�����ӣ��������Ӿ���NԪ�ؼ۵��ӣ���۵��ӹ����ʾʽΪ��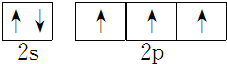 ��
��
�ʴ�Ϊ��1s22s22p63s23p63d104s24p1��4���������壻ԭ�ӣ� ��
��
��2���٦Ҽ�������ת���м�������ת�����Ծ��ЦҼ����ص㣬�ʴ�Ϊ���ң�
��Ԫ�صķǽ�����Խǿ����縺��Խǿ��ͬһ�����У�Ԫ�صĵ縺������ԭ���������������������C��N��H��O����Ԫ�صĵ縺����С�����˳��ΪH��C��N��O��������̼������ÿ����ԭ�Ӻ�̼ԭ���γɹ���˫�������Զ�����̼�ĵ���ʽΪ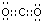 ��ԭ�Ӹ�����ȼ۵�������ȵ���Ϊ�ȵ����壬����N��O�γɺ�CO2�ĵȵ��������ʵĻ�ѧʽΪN2O��
��ԭ�Ӹ�����ȼ۵�������ȵ���Ϊ�ȵ����壬����N��O�γɺ�CO2�ĵȵ��������ʵĻ�ѧʽΪN2O��
�ʴ�Ϊ��H��C��N��O��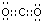 ��N2O��
��N2O��
�۸���ͼƬ֪��a�к��к��������b�к�����λ�����ʴ�Ϊ��a�к��к��������b�к�����λ����
 ��
���ʴ�Ϊ��1s22s22p63s23p63d104s24p1��4���������壻ԭ�ӣ�
 ��
����2���٦Ҽ�������ת���м�������ת�����Ծ��ЦҼ����ص㣬�ʴ�Ϊ���ң�
��Ԫ�صķǽ�����Խǿ����縺��Խǿ��ͬһ�����У�Ԫ�صĵ縺������ԭ���������������������C��N��H��O����Ԫ�صĵ縺����С�����˳��ΪH��C��N��O��������̼������ÿ����ԭ�Ӻ�̼ԭ���γɹ���˫�������Զ�����̼�ĵ���ʽΪ
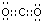 ��ԭ�Ӹ�����ȼ۵�������ȵ���Ϊ�ȵ����壬����N��O�γɺ�CO2�ĵȵ��������ʵĻ�ѧʽΪN2O��
��ԭ�Ӹ�����ȼ۵�������ȵ���Ϊ�ȵ����壬����N��O�γɺ�CO2�ĵȵ��������ʵĻ�ѧʽΪN2O���ʴ�Ϊ��H��C��N��O��
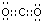 ��N2O��
��N2O���۸���ͼƬ֪��a�к��к��������b�к�����λ�����ʴ�Ϊ��a�к��к��������b�к�����λ����
���������⿼�������ʽṹ�����ʣ���ȷ���ʽṹ�ǽⱾ��ؼ����ṹ�������ʣ�֪���縺�Դ�С���жϷ���������ʽ����д�����ѶȲ���

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ
 ��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
 Al��OH��3+3HCl
Al��OH��3+3HCl