��Ŀ����
����̼������ͭ��һ���¶��·�Ӧʱ������ͭ�ɱ���ԭΪCu2O��Cu���ֽ�2.00g C�� 16.0g CuO�Ļ���������������һ��ʱ���,�����ɵ�����ͨ�������ijΜ[ʯ��ˮ�����ռ���1.12 L����(��״����,���ɳ���������Ϊ5.00 g������˵���������
A.��Ӧ��Ĺ��������л�����̼
B.��Ӧ��Ĺ���������Cu������Ϊ12.8 g
C.��Ӧ��Ĺ������������FΪ14.4 g
D.��Ӧ��Ĺ�������������������ʵ���Ϊ0.05mol
B
��������
���������A��1.12L������CO�����ʵ�����1.12L��22.4L/mol��0.05mol��5.00g������̼��ƣ����ʵ�����5.00g��100g/mol��0.05mol����CO��̼�����̼Ԫ�ص�����֮���ǣ�0.05mol��0.05mol����12g/mol��1.2g��2.00g������̼����������Ӧ��Ĺ��������л�����̼��A��ȷ��B���跴Ӧ��Cu2O��Cu�����ʵ����ֱ���x��y������ݵ��ӵ�ʧ�غ��֪2x��2y��2��0.05mol��4��0.05mol��0.3mol������ͭԭ���غ��֪2x��y��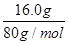 ��0.2mol�����x��0.05mol��y��0.1mol������ ��Ӧ��Ĺ���������Cu������Ϊ0.1mol��64g/mol��6.4g��B����ȷ��C����Ӧ��Ĺ�������������Ϊ2.00g��1.20g��6.4g��0.05mol��144g/mol��14.4
g��C��ȷ��D���������Ϸ�����֪��������ͭ�����ʵ�����0.05mol��D��ȷ����ѡB��
��0.2mol�����x��0.05mol��y��0.1mol������ ��Ӧ��Ĺ���������Cu������Ϊ0.1mol��64g/mol��6.4g��B����ȷ��C����Ӧ��Ĺ�������������Ϊ2.00g��1.20g��6.4g��0.05mol��144g/mol��14.4
g��C��ȷ��D���������Ϸ�����֪��������ͭ�����ʵ�����0.05mol��D��ȷ����ѡB��
���㣺����̼������ͭ��Ӧ���йؼ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A����Ӧ��Ĺ��������л�����̼ | B����Ӧ��Ĺ���������Cu������Ϊ12.8 g | C����Ӧ��Ĺ��������������Ϊ14.4 g | D����Ӧ��Ĺ�������������������ʵ���Ϊ0.05 mol |