��Ŀ����
����Ŀ������п�̸ɵ�����й�������ȣ���������뻷�����������Σ����ij��ѧ��ȤС����������´����������շϵ���еĸ�����Դ��

(1)ʯī�л�ѧ������Ϊ______________���ڵ���е�����Ϊ_________________
(2) ����п�̸ɵ�صĸ�����ӦΪ_________________________
(3) ����п�̸ɵ���ڷŵ���̲���MnOOH��д��������Ӧʽ_____________
(4)�������60����ˮ�ܽ⣬Ŀ����_____________________��
(5)����A������Ϊ______________��
(6)ͭñ�ܽ�ʱ����H2O2��Ŀ����_______________________(�û�ѧ����ʽ��ʾ)
���𰸡����ۼ�(�Ǽ��Թ��ۼ�)�� ���� Zn-2e-=Zn2+ MnO2+e-+H2O=MnOOH+OH- �ӿ��ܽ����� ���� Cu��H2O2��H2SO4��CuSO4��2H2O
��������
�Ͼɸɵ�غ���ͭ��ʯī�����������Լ������ȣ��������60�����ܽ⣬���ˣ���Һ�к����Ȼ�泥�������Ũ�����ᾧ�ɵõ��Ȼ�茶��壻ͭ��ϡ�����ڹ����������÷���������ԭ��Ӧ��������ͭ������п���û���ͭ�����˷��룬����п��Һ���տ�����������п������п��ұ���ɵõ�п���ݴ˽��
(1) ʯī����Ϊ��Ͼ��ͣ��Dz�״�ṹ����ÿһ���ڣ�ÿ��̼ԭ��������3��̼ԭ���Թ��ۼ�����γ���������״�ṹ��ʯī���е����ԣ��ڵ�������缫�������ã�
�ʴ�Ϊ�����ۼ�(�Ǽ��Թ��ۼ�)�����磻
(2) ����п�̸ɵ�صĸ���Ϊп��������ӦΪZn-2e-=Zn2+��
�ʴ�Ϊ��Zn-2e-=Zn2+��
(3) �ڼ���п��ԭ����У�Zn��ʧ���������������������������������϶������̵õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪMnO2+e-+H2O=MnOOH+OH-��
�ʴ�Ϊ��MnO2+e-+H2O=MnOOH+OH-��
(4) �����ʵ������¶ȣ��ɴٽ��ܽ⣬
�ʴ�Ϊ���ӿ��ܽ����ʣ�
(5) ���벻���Թ������Һ���ù��˵ķ��������Բ���A�������ǹ��ˣ�
�ʴ�Ϊ�����ˣ�
(6) ���������£�˫��ˮ�ܽ�ͭ��������ͭ���ӷ�Ӧ�Ļ�ѧ����ʽΪ��Cu��H2O2��H2SO4��CuSO4��2H2O��
�ʴ�Ϊ��Cu��H2O2��H2SO4��CuSO4��2H2O��

����Ŀ�����Ĺ̶�һֱ�ǿ�ѧ���о�����Ҫ���⣬�ϳɰ������˹��̵��Ƚϳ���ļ�������ԭ��ΪN2 (g)+3H2 (g)![]() 2NH3(g)��
2NH3(g)��
(1)��֪ÿ�ƻ�1mol�йػ�ѧ����Ҫ���������±���
H-H | N-H | N-N | N��N |
435.9KJ | 390.8KJ | 192.8KJ | 945.8KJ |
(1)��Ӧ���������_________(�>���� ��<��)���������������
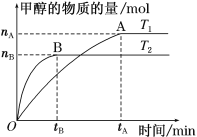
(2)��һ���¶��¡���2L�ܱ������м���2 molN2��6 mol H2�����ʵ��Ĵ��������£�������Ӧ N2 (g)+3H2 (g)![]() 2NH3(g)��10min��ﵽƽ�⣬��ʱʣ��4.5mol H2��
2NH3(g)��10min��ﵽƽ�⣬��ʱʣ��4.5mol H2��
������������˵���˷�Ӧ�ﵽƽ��״̬����________________________��
a����������ѹǿ���� b��v(H2)����v(H2)���� c��N2��H2��Ũ�����
d�� 2 mol NH3���ɵ�ͬʱ��3 moH��H������ e��NH3��Ũ�Ȳ��ٸı�
��0��10 min�ڵ�ƽ����Ӧ����v(H2) ��______mol/(Lmin)��10��ĩNH3��Ũ����______mol/L��N2 �ĵ����ʵ���________mol
��ij�¶�ʱ����һ��2L���ܱ�������X��Y��Z�����������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ���ݴ˻ش�

(1)�÷�Ӧ�Ļ�ѧ����ʽΪ_______________________��
(2)�ӿ�ʼ��2min��Z��ƽ����Ӧ����Ϊ____________mol/(L��min)��
(3)�ı��������������Լӿ컯ѧ��Ӧ���ʵ���_________��
A�������¶� B����С����X�����ʵ��� C����Сѹǿ D����������Z�����ʵ��� E����С�ݻ�
F��ʹ��Ч�ʸ��ߵĴ���
(4)�÷�Ӧ����Ϊ���ȷ�Ӧ����������Ϊ��������(��������Ƚ���)����ƽ������ʱ�佫______��
a���ӳ� b������ c������ d����ȷ��